碳酸钠和氯化钙对软锰矿阳离子浮选中方解石的抑制作用
来源期刊:中国有色金属学报(英文版)2017年第8期
论文作者:Shima RAHIMI Mehdi IRANNAJAD Akbar MEHDILO
文章页码:1831 - 1840
关键词:软锰矿;方解石;浮选;抑制;碳酸钠;氯化钙
Key words:pyrolusite; calcite; flotation; depressant; sodium carbonate; contact angle
摘 要:在软锰矿阳离子浮选中添加十二胺,采用浮选实验、红外光谱分析、接触角测量和zeta电位测试等手段研究碳酸钠和氯化钙对方解石矿物的抑制作用。微浮选实验结果表明,这两种抑制剂都能明显地抑制方解石的浮选。而且,碳酸钠作为活化剂,能增加软锰矿的浮选性能。浮选实验和接触角测试结果表明,碳酸钠对方解石的选择性抑制作用要比氯化钙强。Zeta电位和红外光谱分析表明,碳酸钠减少了方解石矿物表面所带的负电荷,从而减少了十二胺在其表面的静电吸附。在pH=7.5,添加2000 g/t十二胺和1500 g/t碳酸钠的浮选条件下,可得到含40% MnO的软锰矿精矿,回收率达71.5%。
Abstract: In the cationic flotation of pyrolusite using dodecyl ammine (DDA), the depressive effect of sodium carbonate and calcium chloride on the calcite mineral was investigated systematically through flotation experiments, FTIR analysis, contact angle measurements and zeta potential tests. The microflotation experiments showed that both depressant agents decrease the flotation recovery of calcite significantly. In addition, sodium carbonate acts as activator agent for pyrolusite, and increases its floatability. The flotation experiments and contact angle measurements indicated that the selective depression effect of sodium carbonate on the calcite mineral is more than that of calcium chloride. As evidenced by zeta potential and FT-IR analysis, sodium carbonate decreases the negative charges on the surface of calcite mineral and subsequently reduces the adsorption of DDA collector through electrostatic forces. At a pH of 7.5, using 2000 g/t DDA and 1500 g/t sodium carbonate, a pyrolusite concentrate containing almost 40% MnO with 71.5% recovery is achieved by carrying out the ore flotation experiments on the tabling pre-concentrate.
Trans. Nonferrous Met. Soc. China 27(2017) 1831-1840
Shima RAHIMI, Mehdi IRANNAJAD, Akbar MEHDILO
Department of Mining and Metallurgical Engineering, Amirkabir University of Technology, Tehran, Iran
Received 11 May 2016; accepted 1 February 2017
Abstract: In the cationic flotation of pyrolusite using dodecyl ammine (DDA), the depressive effect of sodium carbonate and calcium chloride on the calcite mineral was investigated systematically through flotation experiments, FTIR analysis, contact angle measurements and zeta potential tests. The microflotation experiments showed that both depressant agents decrease the flotation recovery of calcite significantly. In addition, sodium carbonate acts as activator agent for pyrolusite, and increases its floatability. The flotation experiments and contact angle measurements indicated that the selective depression effect of sodium carbonate on the calcite mineral is more than that of calcium chloride. As evidenced by zeta potential and FT-IR analysis, sodium carbonate decreases the negative charges on the surface of calcite mineral and subsequently reduces the adsorption of DDA collector through electrostatic forces. At a pH of 7.5, using 2000 g/t DDA and 1500 g/t sodium carbonate, a pyrolusite concentrate containing almost 40% MnO with 71.5% recovery is achieved by carrying out the ore flotation experiments on the tabling pre-concentrate.
Key words: pyrolusite; calcite; flotation; depressant; sodium carbonate; contact angle
1 Introduction
The significant growth in steel production has recently increased the demands for manganese products. Nowadays, by depletion of the high-grade deposits, considerable attention is being directed to the beneficiation of low-grade manganese ores [1]. The gravity separation methods such as sink-float, jigging and tabling, and high-intensity magnetic separation are usually used to upgrade manganese ores [2,3]. These physical methods are not effective techniques for beneficiation of low grade manganese ores in which the manganese minerals are finely disseminated inside gangue minerals. The froth flotation is an effective and favorable method for processing these kinds of ores [4]. In other words, flotation is one of the developed processes for the beneficiation of lower grade and more refractory resources especially in fine size fractions [2,3]. The flotation reagent plays a critical role in the selective separation process [4]. FUERSTENAU and RICE [5] studied the flotation response of pure pyrolusite with sodium oleate, sodium dodecyl sulfonate and dodecyl ammonium chloride. Sodium or potassium oleate, oleic acid, dodecyl sulfonate, dodecyl sulfate, 2-ethylhexyl sulfosuccinate, dodecylbenzene sulfonate and octyl hydroxamate have been also used as collector for flotation of manganese minerals [6-8]. In these works, the flotation behavior of pure pyrolusite and other manganese oxide minerals has been investigated, and their flotation from gangue minerals has not been considered significantly [5-8]. Calcite is one of the most important gangue minerals in the oxide ores which should be depressed in the flotation process [9-13]. Aluminum sulfate (Al2(SO4)3·12H2O), aluminum chloride, hexamethaphosphate (SH), sodium fluoride, quebracho, starch, sodium silicate and carboxy methyl cellulose (CMC) have been used as a depressant for calcite [12-14]. Aluminum and other polyvalent salts together with silicate have been used in the flotation of scheelite and apatite from carbonaceous gangue [14]. Quebracho was used as a depressant in the separation of hematite from calcite, uranium oxide from gangue and manganese oxide from gangue [14]. Hexametha- phosphate was applied for depressing of calcite in the cationic flotation of smithsonite [11]. Sodium silicate was used as a depressant for calcite in the cationic flotation of pyrolusite by Armac C [15]. In the flotation of scheelite and calcite using dodecylammonium chloride as collector, the use of calcium chloride had a depression effect on calcite flotation [17].
Amir Cheragah deposit (located 82 km northwest of Tabriz, Iran) known as a low grade manganese ore contains calcite as the main gangue mineral (75%-78%) and pyrolusite as the main valuable phase (almost 17%) [16]. In this ore, the fine liberation degree of pyrolusite and its dissemination within the gangue minerals make it difficult to beneficiate the ore by physical methods. In this work, the selective separation of pyrolusite from Amir Cheragah ore by the froth flotation method is investigated. Thus, the most important aim of this work is the investigation of depression effects of sodium carbonate and calcium chloride on calcite in the cationic flotation of pyrolusite.
2 Experimental
2.1 Materials
2.1.1 Sample
The ore sample and purified samples of pyrolusite and calcite taken from Amir Charagah deposit located 82 km from Tabriz, Iran, were used in this work. The purified samples of pyrolusite and calcite were used in the microflotation experiments. For preparation of purified phases, the hand-picked samples taken from the deposit were crushed and ground to less than 150 μm and were then sieved to achieve the 45-150 μm size fraction. The material with this size fraction was processed using a shaking table several times, and the purified phases were obtained. The chemical composition of the purified and ore samples determined is presented in Table 1. Figure 1 shows the XRD patterns of the purified pyrolusite and calcite. These results show that the purified samples are essentially composed of their minerals. Also, based on the XRD and XRF analyses, the ore contains 13.8% MnO which implies about 17% pyrolusite. Almost 79% and 3%-4% of the ore is formed by calcite and quartz, respectively.
2.1.2 Reagents
The flotation reagents are listed in Table 2. All reagents are of analytical grade. Distilled water was used in all experiments.

Fig. 1 XRD patterns of purified samples
Table 1 Chemical composition of purified and ore samples (mass fraction, %)
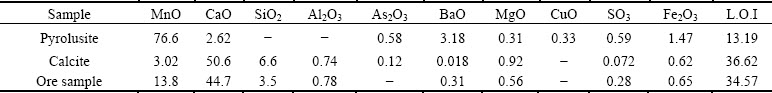
Table 2 Reagents used in flotation experiments
2.2 Methods
2.2.1 Material characterization
The phase analysis or XRD patterns of the samples were carried out with the XPERT MPD diffractometer equipped with Cu Kα radiation. X-ray fluorescence (XRF, Philips X Unique II) was used to determine the chemical composition of the samples.
2.2.2 Microflotation experiments
The microflotation experiments were performed in a 300 mL Hallimond tube at a constant air flow rate of 500 mL/min. In each test, 2 g of purified pyrulosite or calcite was added to the double distilled water and conditioned at the desired pH for 3 min. Then, the collector was added and the suspension was conditioned for 7 min. In the experiments carried out to investigate the effects of depressant agents, these reagents were added to the solution before the collector, with 5 min conditioning time. The prepared pulp was then transferred to the Hallimond tube and flotation was carried out for 4 min. After the flotation tests, the concentrate and tailing were filtered, dried, and weighed. These experiments were carried out at different pH and reagent dosages.
2.2.3 Ore flotation experiments
After grinding and desliming, 300 g of the ore sample with a 20-150 μm size fraction was subjected to the flotation experiments carried out in a 1 l Denver cell with a solid percentage of 25%-30% (mass fraction). After mixing and adjusting the pH of the pulp in the range of 7-7.5 for 4 min, the depressant was added and conditioned for 5 min. Then slurry was conditioned with collector for 5 min and finally, pine oil (100 g/t) was added with a conditioning time of 2 min. The froth collection was performed for 2 min, after which the froth phase was brightened. The flotation concentrate and tailing were filtered, dried and weighed, and analyzed by XRF.
2.2.4 Zeta potential measurement
The zeta potential was measured for purified samples (ground under 5 μm) in the suspension by a Malvern instrument (Nano ZS, ZEN3600, UK) according to its manual procedure. The suspension was prepared by adding 50 mg of purified samples to 100 mL of distilled water containing 1×10-3 mol/L KCl as a supporting electrolyte. The suspensions were stirred for 15 min during which the suspension pH was measured. The pH was adjusted using either NaOH or H2SO4. The zeta potential was also measured in the presence of collector and depressant agents.
2.2.5 Contact angle measurement
The contact angle of distilled water on polished sections of calcite and pyrolusite was measured using the sessile drop method in various concentrations of sodium carbonate and calcium chloride as depressant. The polished section was prepared by polishing various grade sandpapers to a mirror-like finishing. The cleaned sample was immersed in 300 mL of distilled water and conditioned at the desired pH for 3 min. Then, the collector was added to the solution at a concentration of 1×10-4 mol/L and conditioned for 7 min. After adding various concentrations of depressant, the solution was conditioned for 5 min. The sample was brought out from the solution and then air-dried at ambient temperature. For measuring the contact angle, a water drop was placed on the sample using a micro syringe. An image was taken by camera, after the drop was in contact with the sample. The contact angle was measured using Image J software. The reported contact angle for each condition is the average of at least five independent measurements. For cleaning the sample and measuring the contact angle at the next concentrations, the surface of the sample was washed with acetone and lukewarm water, and dried at ambient temperature.
2.2.6 FTIR measurement
The FT-IR analyses were performed by NEXU670 FT-IR (Nicolet Corporation, USA). The conditioning of suspension was carried out on the mineral sample ground under 0.015 mm similar to the flotation experiments. In the quantitative analysis, the ratio of KBr to the sample was 300:1 (w/w).
3 Results and discussion
3.1 Microflotation
3.1.1 Effect of pH
The flotation recovery of purified pyrolusite and calcite using 1.0×10-4 mol/L dodecyl ammine (DDA) as a function of pH values is shown in Fig. 2. The flotation recovery of both minerals increases gradually by increasing the pH value. The maximum flotation recoveries of pyrolusite and calcite are 89% and 82% which are achieved at pH values of 8 and 10, respectively. The further increase in pH decreases their flotation recoveries. The maximum difference between the flotation recoveries of pyrolusite and calcite occurring at a pH of 7-7.5 is almost 20%. At this condition, the flotation recoveries of pyrolusite and calcite are 78.7% and 58.2%, respectively. Thus, for achieving the selective flotation of pyrolusite, the use of a suitable depressant agent for preventing the calcite flotation is necessary.
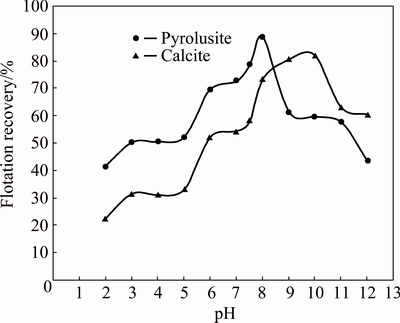
Fig. 2 Flotation recovery of pyrolusite and calcite as a function of pH value (dodecyl ammine: 1.0×10-4 mol/L)
3.1.2 Effect of collector dosage
Figure 3 shows the effect of DDA dosages on the floatability of pyrolusite and calcite at pH of 7.5. The flotation recovery of pyrolusite is more sensitive to the collector dosage than calcite. At low dosages, the recovery of pyrolusite increases with a small increase in DDA dosage. The maximum flotation recovery (78.7%) of pyrolusite is obtained using 1×10-4 mol/L DDA. At this condition, almost 58.2% of calcite is floated. The further increase in DDA dosage does not change the flotation recoveries significantly. The decrease of flotation recovery at high dosages can be attributed to the hydrophobic association of chain-chain interactions through van der Waals forces [18]. While at low dosages, the higher flotation recovery of pyrolusite can be due to the adsorption of cationic collectors through electrostatic interactions. However, in the entire range of collector dosages, the flotation recovery of calcite is lower than that of pyrolusite but it is not low enough to avoid the use of depressant agent. The lower flotation recovery of calcite in comparison to pyrolusite can be due to the lower amount of negative charge on the surface of calcite which prevents the more adsorption of the DDA collector.

Fig. 3 Flotation recovery of pyrolusite and calcite as a function of collector dosage (pH=7.5)
3.1.3 Effect of depressant reagent
Figure 4 shows the effect of sodium carbonate and calcium chloride as calcite depressants on the flotation recovery of pyrolusite and calcite using 1.0×10-4 mol/L DDA at pH of 7.5. As seen from Fig. 4(a), by increasing the dosage of sodium carbonate, the flotation recovery of calcite decreases, while the floatability of pyrolusite improves slightly. This means that the sodium carbonate acts as a depressant agent for calcite and activation agent for pyrolusite. The use of 1.0×10-4 mol/L sodium carbonate decreases the recovery of calcite from 58.2% to 13.8% while it enhances the pyrolusite floatability from 78.7% to 90.5%. The maximum difference between the flotation recoveries achieved using 1.0×10-4 mol/L sodium carbonate is almost 77%. Figure 4(b) indicates that the use of calcium chloride suddenly stops the calcite flotation while it slowly decreases the flotation recovery of pyrolusite. The maximum decrease in the flotation recovery of calcite occurs using 5×10-5 mol/L calcium chloride. At this dosage, the flotation recoveries of calcite and pyrolusite are 10.8% and 76.2%, respectively. Thus, 1.0×10-4 mol/L and 5.0×10-5 mol/L were considered as optimal dosages of sodium carbonate and calcium chloride, respectively, in the next experiments.
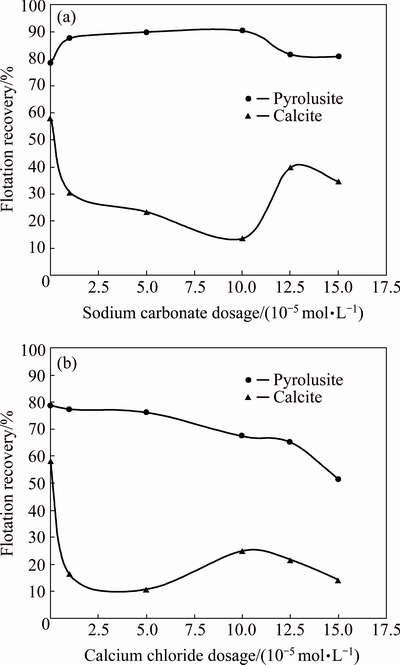
Fig. 4 Effect of sodium carbonate and calcium chloride concentration as depressant on flotation recovery of pyrolusite and calcite (dodecyl ammine: 1.0×10-4 mol/L; pH=7.5)
The flotation recoveries of pyrolusite and calcite as a function of pH values in the presence and absence of sodium carbonate and calcium chloride are shown in Figs. 5 and 6, respectively. As seen from Fig. 5, in strong acidic solutions, sodium carbonate increases the flotation recovery of calcite while it depresses the calcite flotation in weak acidic and alkaline solutions. The maximum depression of calcite flotation occurs using 1.0×10-4 mol/L sodium carbonate at a pH of 7.5 where the flotation recovery of calcite decreases from 57.2% to 13.7%. An important point that should be noted is that the sodium carbonate acts as an activator agent and improves the flotation recovery of pyrolusite in all pH ranges from 2 to 12. Thus, at pH=7.5 using 1.0×10-4 mol/L sodium carbonate the differences between the flotation recoveries of pyrolusite and calcite increase from 20% to almost 77%.
As observed from Fig. 6, the use of 5.0×10-5 mol/L calcium chloride decreases the calcite floatability in a wide pH range. The maximum decrease occurs at a pH of 7.5 where the flotation recovery of calcite reduces from 57.2% to 10.8%. At this condition, the flotation recovery of pyrolusite with a slight decrease reaches from 78.7% to 75.6%. Therefore, using calcium chloride the maximum differences between the flotation recoveries of pyrolusite and calcite improves from 20% to 65.4%.
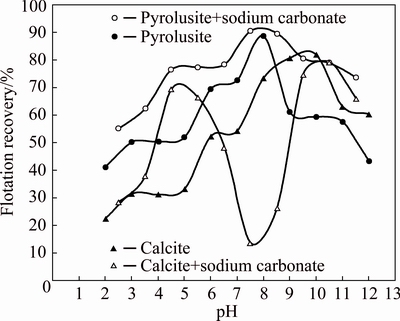
Fig. 5 Flotation recovery of pyrolusite and calcite as a function of pH value in presence and absence of sodium carbonate (dodecyl ammine: 1.0×10-4 mol/L; sodium carbonate: 1.0×10-4 mol/L)
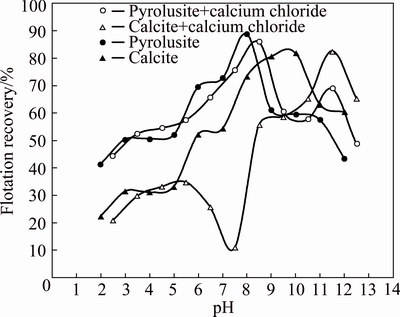
Fig. 6 Flotation recovery of pyrolusite and calcite as a function of pH values in presence and absence of calcium chloride (dodecyl ammine: 1.0×10-4 mol/L; calcium chloride: 5.0×10-5 mol/L)
Table 3 Effect of depressant concentration on pyrolusite flotation (dodecyl ammine: 1.0×10-4 mol/L and pH=7.5)

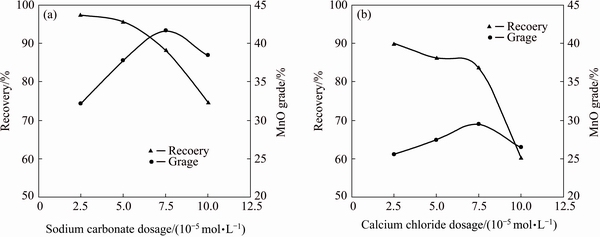
Fig. 7 MnO grade and recovery in flotation concentrate using different dosages of sodium carbonate and calcium chloride (dodecyl ammine: 1.0×10-4 mol/L; pH=7.5)
3.1.4 Microflotation experiments on mixture of both minerals
To investigate the effect of sodium carbonate and calcium chloride as depressant agents on separation of pyrolusite from calcite, the flotation experiments were carried out on the mixture of pure pyrolusite (20%) and calcite (80%, mass fraction). These experiments were carried out using 1.0×10-4 mol/L dodecyl ammine and different dosages of depressant agents at pH=7.5. The results are presented in Table 3 and Fig. 7. The separation efficiency (SE) was calculated by Eq. (1) [19]. The increase of both depressants dosage increases the depression of calcite, and results in the improvement of MnO grade and separation efficiency (SE) in the flotation concentrate. By further increasing the depressants dosage, some of the pyrolusite is also depressed and the MnO grade, recovery and SE decrease. For both depressants, the optimal results with the maximum amounts of separation efficiencies (SE) are achieved at 7.5×10-5 mol/L depressants. When 7.5×10-5 mol/L sodium carbonate is used, a pyrolusite concentrate with 41.7% MnO and 88.3% recovery is obtained while using 7.5×10-5 mol/L calcium chloride a concentrate containing 29.5% MnO with 83% recovery is achieved. These results show that sodium carbonate acts as depressant agent for calcite more effectively than calcium chloride.
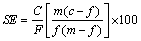 (1)
(1)
where C is the mass of concentrate, F is the mass of feed, c is the MnO content in the concentrate, f is the MnO content in the feed and m is the theoretical content of MnO in pyrolusite.
3.2 Ore flotation
For selecting the optimal dosages of reagents, a number of experiments were carried out on the ore sample (20-150 μm) using different levels of collector and depressant at a pH of 7.5. The optimal results are given in Table 4. Using 2000 g/t DDA without any depressant agent, a pyrolusite concentrate with 17.2% MnO and 62.6% recovery is produced. When 1500 g/t sodium carbonate is used as a depressant agent, the MnO grade and recovery in the pyrolusite concentrate increases to 22.7% and 77.4%, respectively. The use of calcium chloride as a depressant agent results in a pyrolusite concentrate containing 20.3% MnO with 71.8% recovery. The MnO content of the concentrates can be improved by cleaning the rougher stage concentrate. However, both depressant agents improve both MnO grade and recovery but these improvements using sodium carbonate are greater than that of calcium chloride. In order to improve the MnO content of the concentrate, some flotation experiments were carried out on the tabling pre-concentrate (Table 4). The tabling tests were conducted on the ore sample with a size of <500 μm. The prepared concentrate after grinding and desliming with a size of 20-150 μm was subjected to the flotation experiments. The results show that by using sodium carbonate as a depressant agent, a pyrolusite concentrate with 39.9% MnO and 71.5% recovery is obtained. When the calcium chloride is used as depressant agent, a pyrolusite concentrate containing 32.5% MnO with 66.4% recovery is produced. These results indicate that a final pyrolusite concentrate is achievable using the combination of tabling and froth flotation.
3.3 Zeta potential
The zeta potential-pH profile for purified pyrolusite and calcite is displayed in Fig. 8. The IEP (isoelectric point) of pyrolusite and calcite is determined at pH 4.8 and 6.7, respectively. As shown in Fig. 8, the negative charge on the surface of pyrolusite is greater than that of calcite mineral. This means that the surface of pyrolusite for electrostatic interaction of DDA collector is more prone than the calcite surface. Figure 9 shows the zeta potentials of pyrolusite and calcite measured in the absence and presence of 1.0×10-4 mol/L DDA and 1.0×10-4 mol/L sodium carbonate at a pH of 7.5. The zeta potentials of the studied pyrolusite and calcite are -18.7 and -11.6 mV, respectively. This shows that the surface charge of pyrolusite is more negative than that of calcite. The zeta potential at this pH increases in the presence of DDA due to the collector physisorption on the surface of both minerals, and reaches +3.6 and -3.9 mV, respectively. This increase is equal to 22.3 and 7.7 mV for pyrolusite and calcite, respectively. Thus, by using DDA the zeta potential of pyrolusite increases more than that of calcite. This means that the physisorption of DDA collector on the surface of pyrolusite is greater and stronger than that of calcite. The zeta potential of both minerals increases in the presence of sodium carbonate. This increase for calcite mineral is more than that of pyroluslte. Thus, the presence of sodium carbonate decreases the negative charge on the surface of calcite, and results in the decrease of electrostatic interaction between calcite and DDA and hence depresses the flotation of calcite.
Table 4 Results of ore flotation experiments
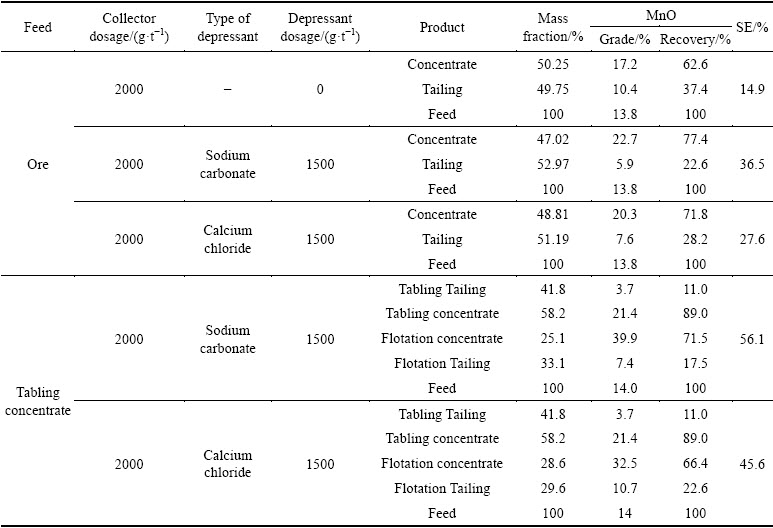
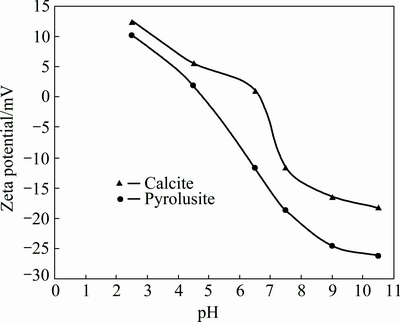
Fig. 8 Zeta potential of purified pyrolusite and calcite as a function of pH
3.4 Contact angle
The hydrophobicity of calcite and pyrolusite conditioned with DDA in the presence of sodium carbonate and calcium chloride was investigated by measuring the contact angle. Figure 10 shows the contact angle as a function of sodium carbonate and calcium chloride dosage in the presence of 1.0×10-4 mol/L DDA at a pH of 7.5. Without any depressant agent, the measured contact angle on the surface of calcite and pyrolusite are 59° and 71°, respectively. This means that using DDA collector, the hydrophobicity of pyrolusite is more than that of calcite mineral. By increasing the dosage of sodium carbonate and calcium chloride, while the contact angle on the surface of pyrolusite conditioned with DDA does not change significantly the contact angle on the surface of the calcite mineral decreases considerably. Using 1.0×10-4 mol/L sodium carbonate and 5.0×10-5 mol/L calcium chloride, the measured contact angle on the surface of calcite decreases from 59° to 39.8° and 41.7°, respectively. This shows that the sodium carbonate and calcium chloride prevent the adsorption of DDA and hence depress the flotation of calcite. These results are in good agreement with the flotation results presented in Fig. 4.

Fig. 9 Zeta potential measurements at pH of 7.5 (2×10-3 mol/L KCl)
Fig. 10 Contact angle on surface of calcite as a function of sodium carbonate and calcium chloride concentration (dodecyl ammine: 1.0×10-4 mol/L; pH=7.5)
3.5 FT-IR analysis
The FT-IR spectra of dodecyl ammine, pure pyrolusite and the adsorption of dodecyl ammine on the surface of pyrolusite at pH of 7.5 are shown in Fig. 11. In the FT-IR spectrum of dodecyl ammine, the bands around 818 and 1564 cm-1 are attributed to N—H flexural vibration bands out of the page. The bands that appear at 1147, 1228, 1321 and 1327 cm-1 are related to the stretching band of C—N. The band around 1371 cm-1 is also related to the —CH3 flexural vibration band [20]. The bands that appear around 2850 and 2920 cm-1 are attributed to —CH2 stretching of acyclic compounds [21-24]. As seen from Fig. 11, some peaks have appeared in the FT-IR spectra of pyrolusite treated with dodecyl ammine at around 1371, 2852 and 2923 cm-1. This indicates that dodecyl ammine has been absorbed on the surface of pyrolusite. The new bonds are not observed in this spectrum. This means that the adsorption of dodecyl ammine on the surface of pyrolusite takes place through the physisorption mechanism.
The FT-IR spectra of calcite, sodium carbonate and calcite treated with sodium carbonate and DDA collector are presented in Fig. 12. The bands related to —CH2 stretching of acyclic compounds located at 2800-3000 cm-1 are observed only in the case of calcite treated with DDA collector. These results indicate that sodium carbonate prevents the adsorption of DDA collector on the surface of the calcite mineral.

Fig. 11 FT-IR spectra of dodecyl ammine (DDA), pure pyrolusite and adsorption of dodecyl ammine on surface of pyrolusite (Pyrolusite + DDA)

Fig. 12 FT-IR spectra of sodium carbonate, calcite, calcite treated with sodium carbonate, calcite treated with DDA collector and calcite treated with sodium carbonate and DDA collector
4 Discussion
The species distribution diagram of DDA plotted according to various solution equilibriums of hydrolysis and dissociation of DDA is shown in Fig. 13 [25]. 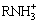 ,
, 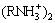 and RNH2·
and RNH2·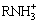 are the most important species which can interact with the surface of minerals in the various pH values. At acidic pH,
are the most important species which can interact with the surface of minerals in the various pH values. At acidic pH, 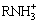 and
and 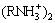 as dominant species cannot react with the positively charged minerals surfaces. At pH above 5 where the pyrolusite surface is negatively charged, these species absorb through electrostatic interaction, and result in the pyrolusite flotation. The maximum flotation recovery of pyrolusite (89%) and calcite (82%) in the pH range of 7-10 can be attributed to the presence of the RNH2·
as dominant species cannot react with the positively charged minerals surfaces. At pH above 5 where the pyrolusite surface is negatively charged, these species absorb through electrostatic interaction, and result in the pyrolusite flotation. The maximum flotation recovery of pyrolusite (89%) and calcite (82%) in the pH range of 7-10 can be attributed to the presence of the RNH2·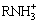 as ion molecular species which are more surface active species to interact electrostatically with the minerals with negative surface charges. As seen from Fig. 13, the decrease of floatability of both minerals in the alkaline solutions can be due to the reduction of the surface active species (
as ion molecular species which are more surface active species to interact electrostatically with the minerals with negative surface charges. As seen from Fig. 13, the decrease of floatability of both minerals in the alkaline solutions can be due to the reduction of the surface active species (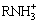 ,
,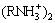 and
and 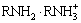 ). At pH of 7.5, the higher flotation recovery of pyrolusite in comparison with calcite is related to the more negative charge on the surface of pyrolusite as evidenced by the zeta potential measurements.
). At pH of 7.5, the higher flotation recovery of pyrolusite in comparison with calcite is related to the more negative charge on the surface of pyrolusite as evidenced by the zeta potential measurements.
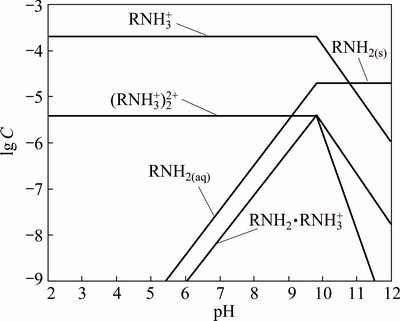
Fig. 13 Distribution diagram of DDA species as a function of pH (total concentration: 1.0×10-4 mol/L)
The high flotation recovery of pyrolusite at low concentrations of collector can be attributed to the electrostatic interactions between DDA collector and the mineral surface [18]. When the collector dosages reach above 10-4 mol/L, the adsorption is ascribed to the hydrophobic association of chain-chain interactions through van der Waals forces [18], which results in the decrease of pyrolusite floatability.
By adding sodium carbonate containing the lattice ions of calcite (Ca2+, 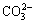 ) [9] to the solution, these ions can adsorb or precipitate at the calcite-water interface [9,26]. These ions can generate some complex ions and compounds which inhibit the interaction between the reagents and calcite through the formation of surface precipitates on the calcite surface [9,10]. It has been proven that the potential determining ions for calcite are Ca2+ and
) [9] to the solution, these ions can adsorb or precipitate at the calcite-water interface [9,26]. These ions can generate some complex ions and compounds which inhibit the interaction between the reagents and calcite through the formation of surface precipitates on the calcite surface [9,10]. It has been proven that the potential determining ions for calcite are Ca2+ and 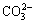 instead of H+ and OH-. The role of pH is only to control carbonate ion speciation [25]. By adding sodium carbonate to the solution, the concentration of
instead of H+ and OH-. The role of pH is only to control carbonate ion speciation [25]. By adding sodium carbonate to the solution, the concentration of 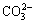 ions increases, and results in the raise of solution pH due to reaction between
ions increases, and results in the raise of solution pH due to reaction between 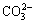 and H+ ions. Sulfuric acid is added to the solution to keep the pH constant. The H+ ions can occupy the surface ≡
and H+ ions. Sulfuric acid is added to the solution to keep the pH constant. The H+ ions can occupy the surface ≡ sites and hence the ≡Ca+ sites on the surface of calcite increase faster than that of surface ≡
sites and hence the ≡Ca+ sites on the surface of calcite increase faster than that of surface ≡ sites. This causes the increase of the zeta potential of calcite, and results in the decrease of the electrostatic interactions of DDA collector ions. Thus, by decreasing the collector adsorption, the hydrophobicity of calcite decreases as evidenced by contact angle measurements, and leads to depression of calcite flotation.
sites. This causes the increase of the zeta potential of calcite, and results in the decrease of the electrostatic interactions of DDA collector ions. Thus, by decreasing the collector adsorption, the hydrophobicity of calcite decreases as evidenced by contact angle measurements, and leads to depression of calcite flotation.
5 Conclusions
1) Using dodecyl amine (DDA) as a cationic collector, the maximum floatability of pyrolusite occurs at pH=7.5-8. In this pH range, the flotation recovery of pyrolusite is higher than that of calcite.
2) Sodium carbonate and calcium chloride have a good depression effect on the calcite flotation. Sodium carbonate acts more effectively than calcium chloride. These depressant agents do not have a significant negative effect on the flotation of pyrolusite.
3) FTIR analyses indicate that the adsorption of DDA collector on the surface of pyrolusite at a natural pH takes place through electrostatic interaction or the physisorption mechanism.
4) The zeta potential measurements show that at pH of 7.5, sodium carbonate decreases the negative surface charges of the calcite more than that of pyrolusite.
5) The contact angle measurements show that sodium carbonate and calcium chloride decrease the contact angle of water drops on the surface of calcite by preventing the more electrostatic interaction between the DDA collector and calcite surface.
6) A pyrolusite concentrate containing almost 40% MnO is achievable using the combination of flotation and gravity separation methods. This combination has higher separation efficiency than those of each method separately.
References
[1] BUCKENHAM M H. Beneficiation of manganese ores with particular reference to the treatment of a low grade ore from Viti Levu, Fiji [J]. New Zealand Journal of Geology and Geophysics, 1961, 4: 136-147.
[2] CROTHERS L A,  J F. Industrial minerals and rocks [M]. SME, 2006: 631-637.
J F. Industrial minerals and rocks [M]. SME, 2006: 631-637.
[3] MISHRA P, MOHAPATRA B, MAHANTA K. Upgradation of low-grade siliceous manganese ore from Bonai-Keonjhar Belt, Orissa, India [J]. Minerals & Materials Characterization & Engineering, 2009, 8: 47-56.
[4] ZHOU Feng, CHEN Tao, YAN Chun-jie, LIANG Huan, CHEN Ting, LI Dan, WANG Qun-ying. The flotation of low-grade manganese ore using a novel linoleate hydroxamic acid [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2015, 466: 1-9.
[5] FUERSTENAU M C, RICE D A. Flotation characteristics of pyrolusite [J]. Trans AIME, 1968, 241: 453-457.
[6] FUERSTENAU M C, HAN K N, MILLER J D. Flotation behavior of chromium and manganese minerals [C]//Proc Arbiter Symposium, Advances in Mineral Processing, 1986: 289-307.
[7] ABEIDU A M. The feasibility of activation of manganese minerals flotation [J]. Trans JIM, 1972, 14: 45-49.
[8] NATARAJAN R, FUERSTENAU D W. Adsorption and flotation behavior of manganese dioxide in the presence of octyl hydroxamate [J]. International Journal of Mineral Processing, 1983, 11: 139-153.
[9] SHI Qing, ZHANG Guo-fan, FENG Qi-ming, OU Le-ming, LU Yi-ping. Effect of the lattice ions on the calcite flotation in presence of Zn(II) [J]. Minerals Engineering, 2013, 40: 24-29.
[10] SHI Qing, FENG Qi-ming, ZHANG Guo-fan, DENG Hong. A novel method to improve depressants actions on calcite flotation [J]. Minerals Engineering, 2014, 466: 186-189.
[11] IRANNAJAD M, EJTEMAEI M, GHARABAGHI M. The effect of reagents on selective flotation of smithsonite–calcite–quartz [J]. Minerals Engineering, 2009, 22: 766-771.
[12] HERNAINZ F, GALVEZ A. The influence of temperature during flotation of celestite and calcite with sodium oleate and quebracho [J]. International Journal of Mineral Processing, 1996, 46: 35-52.
[13] HERNAINZ F, CALERO M, Influence of quebracho and sodium silicate on flotation of celestite and calcite with sodium oleate [J]. International Journal of Mineral Processing, 1993, 37: 283-298.
[14] BULATOVIC S M. Handbook of flotation reagents [M]. Vol. 1. Elsevier Science & Technology, 2007.
[15] MEHDILO A, IRANNAJAD M. Evaluation of pyrolusite flotation behavior using a cationic collector [J]. Journal of Mining Science, 2014, 50: 982-993.
[16] ARNOLD R, BROWNBILL E E, IHLE S W. Hallimond tube flotation of scheelite and calcite with amines [J]. International Journal of Mineral Processing, 1978, 5: 143-152.
[17] MEHDILO A, IRANNAJAD M, BAZDID B. Separation of pyrolusite from calcite by anionic flotation method [J]. Journal of Separation Science and Engineering, 2013, 1: 69-81.
[18] JIANG Hao, XU Long-hua, HU Yue-hua, WANG Dian-zuo, LI Chang-kai, MENG Wei, WANG Xing-jie. Flotation and adsorption of quaternary ammonium cationic collectors on diaspore and kaolinite [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 2528-2534.
[19] GAUDIN A M. Flotation [M]. 2nd ed. New York: McGraw-Hill, 1957.
[20] PAVIA D, LAMPMAN G, KRIZ G, VYVYAN J. Introduction to Spectroscopy [M]. 4th ed. Department of Chemistry, Western Washington University, Bellingham, Washington, 2009.
[21] THISTLETHWAITE P J, HOOK M S. Diffuse reflectance Fourier transform infrared study of the adsorption of oleate/oleic acid onto titania [J]. Langmuir, 2000, 16: 4993-4998.
[22] PECK A S, RABY L H, WADSWORTH M E. An infrared study of the flotation of hematite with oleic acid and sodium oleate [J]. Transactions of the American Institute of Mining, 1966, 235: 301-307.
[23] GUAN Feng, ZHONG Hong, LIU Guang-yi, ZHAO Sheng-gui, XIA Liu-yin. Flotation of aluminosilicate minerals using alkylguanidine collectors [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 228-234.
[24] XIA Liu-yin, ZHONG Hong, LIU Guang-yi, HUANG Zhi-qiang, CHANG Qing-wei, LI Xin-gang. Comparative studies on flotation of illite, pyrophyllite and kaolinite with Gemini and conventional cationic surfactants [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 446-453.
[25] GAO Zhi-yong, SUN Wei, HU Yue-hua. New insights into the dodecylamine adsorption on scheelite and calcite: An adsorption model [J]. Minerals Engineering, 2015, 79: 54-61.
[26] HEBERLING F, TRAINOR T P,  J, ENG P, DENECKE M A, BOSBACH D. Structure and reactivity of the calcite–water interface [J]. Journal of Colloid and Interface Science, 2011, 354: 843-857.
J, ENG P, DENECKE M A, BOSBACH D. Structure and reactivity of the calcite–water interface [J]. Journal of Colloid and Interface Science, 2011, 354: 843-857.
Shima RAHIMI, Mehdi IRANNAJAD, Akbar MEHDILO
Department of Mining and Metallurgical Engineering, Amirkabir University of Technology, Tehran, Iran
摘 要:在软锰矿阳离子浮选中添加十二胺,采用浮选实验、红外光谱分析、接触角测量和zeta电位测试等手段研究碳酸钠和氯化钙对方解石矿物的抑制作用。微浮选实验结果表明,这两种抑制剂都能明显地抑制方解石的浮选。而且,碳酸钠作为活化剂,能增加软锰矿的浮选性能。浮选实验和接触角测试结果表明,碳酸钠对方解石的选择性抑制作用要比氯化钙强。Zeta电位和红外光谱分析表明,碳酸钠减少了方解石矿物表面所带的负电荷,从而减少了十二胺在其表面的静电吸附。在pH=7.5,添加2000 g/t十二胺和1500 g/t碳酸钠的浮选条件下,可得到含40% MnO的软锰矿精矿,回收率达71.5%。
关键词:软锰矿;方解石;浮选;抑制;碳酸钠;氯化钙
(Edited by Sai-qian YUAN)
Corresponding author: Mehdi IRANNAJAD; E-mail: iranajad@aut.ac.ir
DOI: 10.1016/S1003-6326(17)60206-1

