
Degradation mechanisms of poly (lactic-co-glycolic acid) films in vitro under static and dynamic environment
HUANG Ying-ying(黄莹莹), QI Min (齐 民), ZHANG Meng(张 萌),
LIU Hong-ze(刘洪泽), YANG Da-zhi(杨大智)
State Key Laboratory of Materials Modification, School of Materials Science and Engineering,
Dalian University of Technology, Dalian 116023, China
Received 10 April 2006; accepted 25 April 2006
Abstract: To understand their degradation mechanisms, PLGA (50:50) polymer films were prepared and eroded in the static and dynamic medium system. The degradation behavior was characterized through weight-average molecular weight change, mass loss, water uptake, etc. The results show that in dynamic system, significant mass loss begins until 10 d while mass loss does not begin until 30 d later, while weight-average molecular weight decreases observably at the beginning, and the appeasable mass loss happens in 20 d in static system, which suggests that the dynamic degradation rate is slower even than degradation in static medium. A mechanism was proposed that specimens in static medium take up water homogeneously and cause the polymer chains to degrade all over the specimen cross sections, which creates free carboxylic acid groups which lead to a decrease of pH value inside the swollen polymer and accelerate degradation of the polymer. While pH value inside polymer keeps constant in dynamic medium because of flowing of simulated medium, which make the hydrolytic cleavage of ester bonds inside specimen delayed.
Key words: poly lactic-co-glycolic acid; hydrolytic degradation; dynamic medium system
1 Introduction
Poly lactic-co-glycolic acid (PLGA) have been extensively used as a controlling release carrier for drug delivery due to their good biocompatibility, biodegradability and mechanical strengths [1, 2]. PLGA is hydrolytically unstable, they degrade by hydrolytic attack of their ester bonds [3], resulting in the formation of lactic and glycolic acids. An important attribute of these polymers is the possibility to modulate the degradation rate of a delivery system by changing, e. g. chemical composition (homo-or copolymers of lactic and glycolic acid) or the physical properties (molecular weight, glass-transition temperature) and consequently to control the drug release [4]. Therefore, the degradation mechanism of polyesters has been drawing much attention [5-9]. Generally, the hydrolytic degradation of polyesters in aqueous media proceeds through random ester bond cleavage in the bulk of the device [6, 10, 11].
However, degradation causes an increase of the number of carboxylic chain ends which are known to autocatalyze the ester hydrolysis [5]. And, only oligomers soluble in the surrounding aqueous medium can escape from the matrix. As the ageing time increases, soluble oligomers close to the surface can leach out before total degradation whereas those which are located well inside the matrix remain entrapped and contribute totally to the autocatalytic effect. Degradation rates depend on the degree of swelling, and probably also on the sequential distribution of chiral and achiral units along the polymer chains [12, 13]. Last but not least, the release of the soluble carboxyl-terminated oligomers depends on their solubility in the surrounding aqueous medium and thus on factors like pH value, ionic strength, temperature and buffering capacity [14]. So surrounding medium is the important factor on the polymer degradation. These surrounding medium are static solution system, but condition inside human body is circulating system and the body fluid is flowing, investigation under dynamic system which can simulate the body fluid circulating system have not been reported. In the present work, a the dynamic simulated system was established (Fig.1).
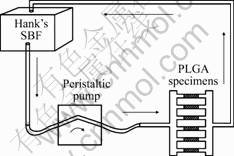
Fig.1 Schomatic diagram of dynamic simulated system
In order to understand degradation mechanisms in these different medium systems, a comparative study on the degradation of PLGA (50∶50) matrix in static and dynamic medium system was performed. The PLGA films were placed in the static medium and dynamic medium respectively, at 37 ℃. The degradation process was monitored by measuring weight-average molecular weigh, mass loss, water uptake and morphology.
2 Experimental
2.1 Specimen preparation
PLGA (50∶50) with weigth-average molecular weight(Mw) of 60 000 was provided by State Key Laboratory of Biomaterials (Shandong, China). 1, 4-dioxane was of analytical purity.
PLGA thin films were prepared by casting 1, 4-dioxane solutions of the copolymer with a concentration of 5%. After solvent evaporation at room temperature, the PLGA films were removed and rolled into a cylinder(5 mm in diameter, 20 mm in length). The cylinder specimens were dried thoroughly at room temperature under vacuum to constant mass, and then weighed.
2.2 Degradation experiment
Specimens for static and dynamic degradation experiments were placed in identical flasks and dynamic circulation system (Fig.1), respectively. Hank’s simulated body fluid (Hank’s SBF) at 37 ℃ was selected. After selected degradation times, specimens were taken out, washed three times with distilled water, and vacuum-dried at room temperature before being subjected to the various analyses. The rate of flowing medium was adjusted to that close to body fluid by changing the pump flux. The dynamic system was cleaned and the medium was refreshed every week.
2.3 Characterization
2.3.1 Weight-average molecular weigh and poly-dispersity
Mw and polydispersity of the PLGA specimens were determined by PL-220 gel permeation chromatography (GPC) using tetrahydrofuran (THF) as eluent at a flow rate of 1.0 mL/min at 40 ℃ and using polystyrene as standards.
2.3.2 Remained mass
The remained mass(x) was calculated by comparing the dry remained mass (md) of the remained specimen after degradation for a predetermined time with the original mass (m0) of the specimen as
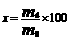 (1)
(1)
2.3.3 Water uptake
Water uptake of the PLGA specimen was calculated according to
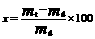 (2)
(2)
where mt and md are the wet and dry mass of the residual specimen after degrading a predetermined time, respectively.
2.3.4 Morphology
Morphology identification of PLGA specimen was performed with a scanning electron microscope (SEM, JSM-5600, operated at 15 kV)
3 Results and discussion
3.1 Results of degradation
The mass and weight-average molecular weight(Mw) of residual polymer in static degradation experiments are shown in Fig.2. Rapid decrease of Mw was seen in static SBF while significant mass loss did not begin until 20 d later. Fig.3 shows that the profiles of PLGA in dynamic system change. Significant decrease of Mw began until 10 d while mass loss did not begin until 30 d later.
Degradation arises from water uptake by the polymers which are shown in Fig.4. Fig. 4 indicates that
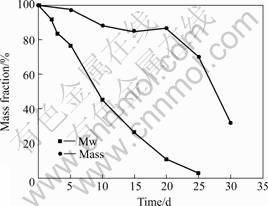
Fig.2 Static degradation of PLGA
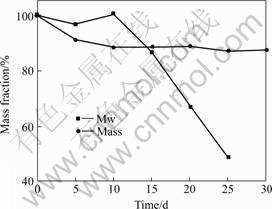
Fig.3 Dynamic degradation of PLGA
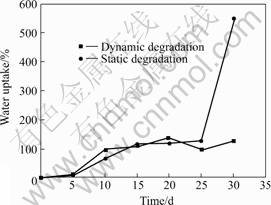
Fig.4 Water uptake of PLGA degradation
the polymers in static and dynamic medium take up an amount of water even before the onset of mass loss is reached, but there was no significant changing from 25 to 30 d contrast between the water uptake of PLGA in dynamic and static degradation.
Fig.5 show that the SEM image of PLGA after the degradation in static and dynamic system. A lot of holes form on surface of static degradation specimen after 15 d and hollow structure forms inside specimen after 25 d, while no significant change on surface of dynamic degradation specimen after 15 d and the surface hole only formed after 35 d which indicates that the dynamic degradation rate is slower even than degradation in static medium.
3.2 Discussion
From the above degradation results, it is very interesting that the dynamic degradation rate is slower even than degradation in static medium. According to difference of solution surrounding surface of the polymers [15, 16], we proposed degradation mechanisms both in static and dynamic conditions as shown in Fig.6.
In static situation Fig.6 (a), autocatalysis arises from the uptake of water by the polymers and then creates carboxylic acid end groups due to the degradation of the polymer [17]. As no swelling fronts are observed, one can assume that the specimens take up water homogeneously and cause the polymer chains to degrade all over the specimen cross sections. This creates free carboxylic acid groups which lead to a decrease of pH value inside the swollen polymer specimen and lead to acceleration of degradation inside the polymer bulk [18]. As the surface of the polymer is kept at neutral pH value , a pH value gradient develops that slows down the degradation of the polymer matrix surface compared to the center. The surface layer breaks at some point when a critical osmotic pressure builds up inside the matrix due to the accumulation of degradation products. And degradation products can not apparently leave the specimen prior to the mass loss onset. Only after a critical degree of degradation is reached, the polymers form a network of pores that allows for the release of monomers and oligomers [19].
The process of dynamic degradation is shown in Fig.6 (b). In dynamic system, water diffusion to inside specimen decreased because of the simulated medium flowing at direction, which makes the hydrolytic cleavage of ester bonds inside specimen delayed. The cleavage of ester bonds occurs preferentially at the surface of the specimen because of probability of the water contact with specimen surface increased due to the medium flowing. Generated carboxylic acid groups did not accumulate and washed out by flowing medium. However, that is not surface degradation [20] because that mass loss of surface degradation starts the beginning of the experiment and the number-average molecular mass almost does not change in a long time. But in our experiment, the mass loss almost does not change in all over 30 d and Mw decreasedstarts until 10 d later. One can assume that dynamic degradation delays bulk degradation. Degradation starts with the absorption of water, followed by the hydrolytic cleavage of ester bond, which generates chain fragments with acidic end groups [21,22]. Less absorption of water make duration of other progress of hydrolysis degradation delayed. After a long period of time this gradient of absorbed water disappears, more hydrolytic cleavage of ester bond take place.
Finally, it should be noticed that the degradation mechanism in static medium would be considerably bulk degradation, while that in dynamic medium would delay bulk degradation because of water diffusion to inside specimen decreasing for the simulated medium flowing at direction.
4 Conclusions
1) Rapid decrease in mass was seen upon in static SBF while significant mass loss does not begin until
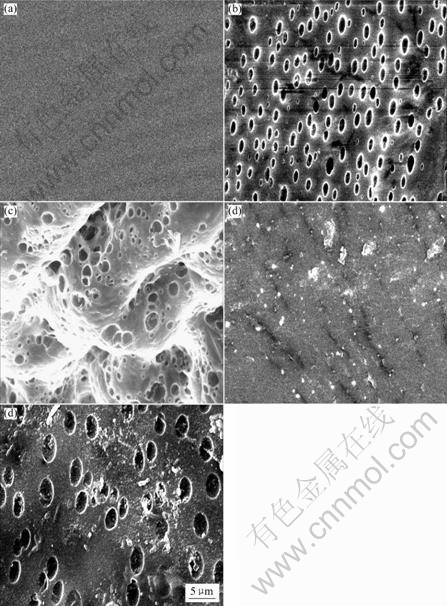
Fig.5 SEM photographs of degrading PLGA (50∶50): (a) Original;(b) Static degradation for 15 d;(c) Static degradation for 25 d;(d) Dynamic degradation for 15 d;(e) Dynamic degradation for 35 d
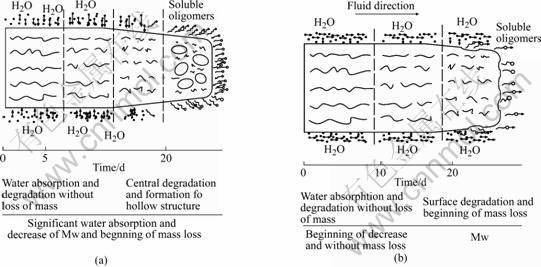
Fig.6 Progress of PLGA degradation: (a) Static degradation; (b) Dynamic degradation
20 d later. Significant decrease in mass was seen upon of dynamic SBF began until 10 d while mass loss does not begin until 30 d later.
2) There are a lot of holes on surface of static degradation specimen after 15 d and hollow structure formed inside specimen after 25 d, while no significant change on surface of dynamic degradation after 15 d and the surface hole only forms after 35 d which indicate that the dynamic degradation rate is slower even than degradation in static medium.
3) The mechanism of water diffusion in polymer bulk is an important factor for polymer degradation. References
[1] PEPPAS N A, HUANG Y, TORRES L M, WARD J H, ZHANG J. Physicochemical foundations and structural design of hydrogels in medicine and biology[J]. Ann Rev Biomed Eng, 2000, 2: 9-27.
[2] JAIN R A. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co -glycolide) (PLGA) devices[J]. Biomaterials, 2000, 21: 2475-2490.
[3] GRIFFITH L G. Polymeric biomaterials[J]. Acta Mater, 2000, 48: 263-277.
[4] WU L B, DING J D. In vitro degradation of three-dimensional porous poly (D,L-lactide -co-glycolide) scaffolds for tissue engineering [J]. Biomaterials, 2004, 25: 5821-5830.
[5] BURKERSRODA F V, SCHEDL L, GOPFERICH A. Why degradable polymers undergo surface erosion or bulk erosion[J]. Biomaterials, 2002, 23: 4221-4231.
[6] SCHLIECKER G, SCHMIDT C, FUCHS S, KISSEL T. Characterization of homologous series of D,L-lactic acid oligomers; a mechanistic study on the degradation kinetics in vitro[J]. Biomaterials, 2003, 24: 3835-3844.
[7] SCHLIECKER G, SCHMIDT C, FUCHS S, WOMBACHER R, KISSEL T. Hydrolytic degradation of poly (lactide-co-glycolide) films: effect of oligomers on degradation rate and crystallinity[J]. International Journal of Pharmaceutics, 2003, 266: 39-49.
[8] BARBOSA M E M, CAMMAS S, APPEL M PONCHEL G. Investigation of the degradation mechanisms of poly(malicacid) esters in vitro and their related bytotoxicities on J774 macrophages [J]. Biomacromolecules, 2004, 5: 137-143.
[9] ESPARTERO J L, RASHKOV I, LI S M, MANOLOVA N, VERT M. NMR Analysis of low molecular weight poly (lactic acid)[J]. Macromolecules, 1996, 29: 3535-3539.
[10] VERT M, SCHWACH G, ENGEL R, COUDANE J. Something new in the field of PLA/GA bioresorbable polymers[J]. J Control Release, 1998, 53(1-3): 85-92.
[11] VERT M, MAUDUIT J, LI S. Biodegradation of PLA/GA polymers: increasing complexity [J]. Biomaterials, 1994, 15 (15): 1209-1213.
[12] LI S M, VERT M. Crystalline oligomeric stereocomplex as intermediate compound in racemic poly (nL-lactic acid) degradation[J]. Polym Int, 1994, 33: 3741.
[13] LI S M, VERT M. Morphological changes resulting from the hydrolytic degradation of stereocopolymers derived from L- and D-lactides [J]. Macromolecules, 1994, 27: 3107-3110.
[14] VERT M. Degradation of polymeric biomaterials with respect to temporary therapeutic applications: tricks and treats[J]. BioDegradable Materials, 1990, 11-37.
[15] LI S M, VERT M. Hydrolytic degradation of the coral/poly(d,llactic acid) bioresorbable material[J]. J Biomater Sci Polym Ed, 1996, 7(9): 817-827.
[16] G?PFERICH A, LANGERR R. Modeling of polymer erosion [J]. Macromolecules, 1993, 26: 4105-112.
[17] LI S M, GARREAU H, VERT M. Structure-property relationships in the case of the degradation of massive aliphatic poly-(a-hydroxy acids) in aqueous media, Part 1[J]. J Mater Sci: Mater Med, 1990, 1: 123-126.
[18] GRIZZI I, GARREAU S, VERT M. Hydrolytic degradation of devices based on poly (DL-lactic acid) size-dependence [J]. Biomaterials, 1995, 16: 305-311.
[19] G?PFERICH A. Polymer bulk erosion. Macromolecules 1997, 30(9): 2598-2604.
[20] G?PFERICH A, LANGER R. The influence of microstructure and monomer properties on the erosion mechanism of a class of polyanhydrides[J]. J Polym Sci Part A: Polym Chem, 1993, 31: 2445-2458.
[21] SHAH S, CHA Y, PITT C. Poly (glycolic acid-co-dl-lactic acid): diffusion or degradation controlled drug delivery[J]. J Control Release, 1992, 18: 261-70.
[22] LI S, VERT M. Biodegradable Polymers: polyesters[A]. Mathiowitz E. Encyclopedia of Controlled Drug Delivery[C]. New York: Wiley, 1999. 71-93.
(Edited by YANG Hua)
Foundation item: Projects(2002AA326010; 2004AA32G110) supported by the High-tech Research and Development Program of China ; Project ( 30470521) supported by the National Natural Science Foundation of China
Corresponding author: QI Min; Tel: +86-411-84708441; Fax: +86-411-84709284; E-mail: minqi@dlut.edu.cn