Dissolution of gold in chalcopyrite-containing cyanide solutions
来源期刊:中南大学学报(英文版)2020年第5期
论文作者:杨玮 张凯 王亚平 龙涛 宛鹤 李慧 王倩
文章页码:1495 - 1502
Key words:chalcopyrite; cyanide; gold; dissolution
Abstract: The gold dissolution in anoxic cyanide solution in the presence of chalcopyrite was studied with a rotating disc electrode. It was found that the presence of chalcopyrite reduced and enhanced the dissolution activity of pure gold in the low and high potential regions, respectively. The dissolution of gold was diffusion-controlled at low potentials (≤177 mV) and low cyanide concentrations (≤980 mg/L); however, above the cyanide concentration of 980 mg/L, the current density of gold decreased and the dissolution of gold changed from diffusion-control to electrochemical reaction-control. At high potentials (>177 mV), gold dissolution was always controlled by diffusion. In cyanide solution containing chalcopyrite, appropriate increase of pH value and temperature could accelerate the dissolution of gold, but high pH value would promote the generation of passivation, which was harmful for the dissolution of gold in cyanide solution.
Cite this article as: YANG Wei, ZHANG Kai, WANG Ya-ping, LONG Tao, WAN He, LI Hui, WANG Qian. Dissolution of gold in chalcopyrite-containing cyanide solutions [J]. Journal of Central South University, 2020, 27(5): 1495-1502. DOI: https://doi.org/10.1007/s11771-020-4385-z.

J. Cent. South Univ. (2020) 27: 1495-1502
DOI: https://doi.org/10.1007/s11771-020-4385-z

YANG Wei(杨玮)1, 2, ZHANG Kai(张凯)1, 2, WANG Ya-ping(王亚平)3, LONG Tao(龙涛)1, 2,
WAN He(宛鹤)1, 2, LI Hui(李慧)1, 2, WANG Qian(王倩)1, 2
1. School of Resources Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, China;
2. Key Laboratory of Gold and Resources in Shaanxi Province, Xi’an 710055, China;
3. School of Foreign Languages, Baoji University of Arts and Sciences, Baoji 721013, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: The gold dissolution in anoxic cyanide solution in the presence of chalcopyrite was studied with a rotating disc electrode. It was found that the presence of chalcopyrite reduced and enhanced the dissolution activity of pure gold in the low and high potential regions, respectively. The dissolution of gold was diffusion-controlled at low potentials (≤177 mV) and low cyanide concentrations (≤980 mg/L); however, above the cyanide concentration of 980 mg/L, the current density of gold decreased and the dissolution of gold changed from diffusion-control to electrochemical reaction-control. At high potentials (>177 mV), gold dissolution was always controlled by diffusion. In cyanide solution containing chalcopyrite, appropriate increase of pH value and temperature could accelerate the dissolution of gold, but high pH value would promote the generation of passivation, which was harmful for the dissolution of gold in cyanide solution.
Key words: chalcopyrite; cyanide; gold; dissolution
Cite this article as: YANG Wei, ZHANG Kai, WANG Ya-ping, LONG Tao, WAN He, LI Hui, WANG Qian. Dissolution of gold in chalcopyrite-containing cyanide solutions [J]. Journal of Central South University, 2020, 27(5): 1495-1502. DOI: https://doi.org/10.1007/s11771-020-4385-z.
1 Introduction
The cyanidation has been applied in industry since 1887. It was widely applied because of its simple process, high recovery rate and strong applicability. However, the slow dissolution rate of gold is still one of the main problems of cyanidation. It has been known that gold cyanidation is an electrochemical reaction [1]. Cyanidation occurs as a result of the two redox half reactions. Anodic dissolution of gold will be oxidized according to the following reaction:
 →
→ (1)
(1)
On the surface of gold, oxygen will be reduced according to the following reaction:
 (2)
(2)
Hence, many researchers used electrochemical methods to study the kinetics of gold. In aerated reagent-grade cyanide solution, KUDRYK et al [2] examined the anodic dissolution of pure gold. They found that the dissolution rate of pure gold was controlled by the diffusion of cyanide ions, whereas JEFFREY et al [3] believed that in aerated ultra-pure cyanide solutions, the dissolution rate of pure gold is very low, so the reaction is chemically controlled rather than controlled by cyanide ion diffusion. MRKUSIC et al [4] reported that the anodic curve of pure gold obtained in the presence of magnetite showed that the rate of gold dissolution could be controlled by the anodic or cathodic reaction, but not by diffusion. GUAN et al [5] reported the dissolution behavior of gold/copper alloys in the aerated cyanide solutions and found that the dissolution rate of gold/copper alloys was partially controlled by the electrochemical reaction but largely by oxygen diffusion to the alloy surface. Moreover, the presence of such impurity ions as heavy metal ions [6, 7] and sulfide ions also has a significant effect on the dissolution of gold in cyanide solution [8].
When gold is extracted from copper-bearing gold ore by cyanidation, the presence of copper minerals will greatly affect the leaching of gold [9-11]. For example, in oxygen-enriched cyanide solutions, the presence of chalcopyrite was found to decrease the leaching rate of gold [12, 13]. This is because most copper minerals are greatly soluble in cyanide solutions [14], and their dissolution consumes large amounts of cyanide and oxygen [15-17], thus reducing the concentration of the free cyanide and oxygen in interaction with gold. As a result, the leaching rate of gold is lowered. In addition, the dissolution of copper sulfide produces a certain amount of sulfide ions [16]. These sulfide ions will adsorb on the gold surface and form a single layer of sulfur [18, 19], and the ions also interact with gold to form Au/Sx or Au2S passivation film [20-22], which will adversely affect the cyanidation of gold. At the same time, most sulfide minerals are semi-conductors. So, the galvanic interactions of copper sulfide and gold are also important factors for the dissolution rate of gold [21, 23].
In order to eliminate the adverse effects of copper minerals, it is often necessary to add a certain amount of leaching aid during the cyanidation of copper-bearing gold ores [24-27]. However, there is a corresponding relationship between these leaching aids and the copper-bearing gold ores. For example, ammonia-based leaching aids have the most efficacious effect on copper- containing gold ore mainly with copper oxide minerals [28, 29]. And in our research, we also found that lead salt leaching aids have better strengthening effect on copper-containing gold ores mainly with copper sulfide minerals [30]. The reason for this is that under different conditions, there are differences in the inhibition mechanism of copper minerals on gold.
Therefore, it is necessary to systematically study the effects of different leaching conditions (cyanide concentration, pH value, stirring speed, and leaching temperature) on the cyanidation of gold in the presence of copper minerals, which will provide guidance for the development of leaching aids of the copper-containing gold ores. This study chose pure gold as the research object to explore the effect of leaching conditions on the dissolution of gold in chalcopyrite-containing cyanide solution.
2 Experimental
2.1 Minerals and reagents
Chalcopyrite used in this study was obtained from Daye Mining, Hubei Province, China. After dry-grinding in an agate mortar, the particle size of each sample was milled to be less than 75 μm, and then they were stored in high-purity nitrogen-filled airtight plastic bags and refrigerated to avoid surface oxidation. The chemical compositions of the samples are listed in Table 1. Quantitative X-ray diffraction (XRD) analysis was also utilized to confirm the mineralogy of the samples (see Figure 1). The results indicate that the samples contained 98.9% chalcopyrite (CuFeS2), 0.82% copper iron sulfide (Cu9Fe9S16) and 0.28% nukundamite ((Cu, Fe)4S4). All the reagents used in the experiment were analytically pure. Deionized water was used throughout all experiments.
Table1 Chemical composition of chalcopyrite (mass fraction, %)


Figure 1 XRD diffraction pattern of chalcopyrite
2.2 Electrochemical test
In the presence of chalcopyrite, the dissolution of gold was studied by using the rotating disc technique. The electrochemical experiments were carried out in an electrochemical cell with gold (99.99% Au) as rotating working electrode of 0.19625 cm2 and platinum electrode (99.99% Pt) as a counter electrode. And a saturated silver chloride electrode is connected with a Luggin tube to the cell as a reference electrode. The cell itself was made of glass and had a capacity of 125 mL. KNO3 was used as an electrolyte with the concentration of 1 mol/L; the pH of the electrolyte was adjusted with NaOH and circulator water bath was applied to achieve better effect of constant temperature. To prevent oxygen from affecting the testing period, the solution was deoxygenated with high purity nitrogen gas (N2 99.999%) before each test. Nitrogen was continuously passed in the solution till the end of the experiment. The oxygen concentration was monitored by Seven2Go Pro S9 oxygen meter. Before each experiment, the gold electrode was polished with 2000-mesh metallographic sandpaper first and then with alumina powder. After that, the gold electrode was washed with dilute nitric acid (CHNO3=0.01 mol/L) and then finally rinsed with deionized water. Gold electrode was immersed in the cyanide solutions,2 cm below the solution surface. All potentials were given with respect to the standard hydrogen electrode (SHE).
3 Results and discussion
3.1 Effect of chalcopyrite on dissolution of gold
The effect of chalcopyrite on dissolution of gold in the cyanide solution is shown in Figure 2 where DO is the dissolved oxygen concentration. The gold open circuit potential was about -600 mV in clean cyanide solution. But when chalcopyrite was present in the cyanide solution, the gold open circuit potential was about -400 mV, showing a positive move. When the overpotential was lower than -200 mV, the current density in the presence of chalcopyrite was lower than that in the absence of chalcopyrite. This indicates that the presence of chalcopyrite inhibits the cyanide dissolution of gold and reduces the dissolution rate of gold in the active dissolution region. However, at high potentials, the chalcopyrite could remove the passivation of gold surface and promote gold dissolution, and in the presence of chalcopyrite, the polarization curve of gold presents a “platform region” in the range of potential 200-500 mV, which is suggesting diffusion. This indicates that the presence of chalcopyrite could inhibit the cyanidation of gold under low potential and activate the cyanidation of gold under high potential. This could be due to the fact that sulfide ions deposit on the gold surface at low potential and form a single layer and thus inhibit the dissolution of gold [19]; the reason of gold cyanide dissolution activated by chalcopyrite under high potential could be similar to that of polysulfide leaching [31].

Figure 2 Gold polarization curve in the absence and presence of chalcopyrite (conditions: scan rate=1.0 mV/s, ρ(NaCN)=490 mg/L, pH=10.5, T=298 K, disc rotation speed=400 r/min, DO=0, chalcopyrite 0.29 g/L)
3.2 Effect of cyanide concentration
The influence of cyanide concentrations on the dissolution rate of gold was studied in the presence of chalcopyrite. The polarization curves in Figure 3 indicate that the gold open circuit potential did not obviously change at different cyanide concentrations. However, the effects of cyanide concentrations on the dissolution of gold were differentiated at the different potential regions. When the potential was under -177 mV and cyanide concentration was below 980 mg/L, the anode polarization curves slightly rise with increasing cyanide concentrations. And after the cyanide concentrations increased above 490 mg/L, the gold anode polarization curves showed a weak current peak near the potential of -150 mV, which marked the occurrence of passivation. Meanwhile, the current peak shifted slightly toward the cathode potential when the cyanide concentration increased to 1470 mg/L, and the anode polarization curve sharply dropped. However, when the potential was beyond -177 mV, the anode polarization curves rose continuously with increasing cyanide concentrations. This experiment showed that at lower potentials, increasing cyanide concentrations could effectively inhibit the adsorption of sulfide ions on gold surface so as to enhance the dissolution rate of gold [20]. However, when cyanide concentrations were beyond 1470 mg/L, on one hand, chalcopyrite dissolution strengthened and produced more sulfide ions;on the other hand, excessive CN- ions would also adsorb on the gold surface and generate the passivation layer of AuCNads which will hinder the dissolution of gold [32].

Figure 3 Effect of cyanide concentration on gold polarization curves: 1-245 mg/L; 2-490 mg/L; 3-700 mg/L; 4-980 mg/L; 5-1470 mg/L (conditions: scan rate=1.0 mV/s, pH=10.5, T=298 K, disc rotation speed=400 r/min, DO=0)
In order to study the effect of cyanide concentration on the dissolution of gold anodes in different potentials, the current density at -226 mV and 166 mV was selected from Figure 3 to investigate the correlation between current density and cyanide concentrations (see Figures 4(a) and (b)). From Figure 4(a), at low cyanide concentrations, the current density increased approximately linearly with the cyanide concentrations. However, when the cyanide concentration was beyond 980 mg/L, the current density decreased remarkably. This indicated that, at low cyanide concentration, the dissolution rate of gold was controlled by the cyanide diffusion, while at high cyanide concentration, the control steps of the dissolution rate of gold changed. Similar results were obtained by YANG [33].

Figure 4 Variation of current density with cyanide concentration at E=-226 mV(a) and E=166 mV (b)
As could be seen from Figure 4(b), when the potential was 166 mV, the anode current density of gold increased almost linearly with the cyanide concentration (R2=0.98), and the excessive cyanide concentration would not reduce the anode current density. This indicated that the anode dissolution rate of gold was controlled by cyanide diffusion at high potential.
3.3 Effect of pH
Figure 5 presents the effect of the pH on the dissolution of pure gold in the presence of chalcopyrite. At the negative potentials region, the results showed that the pH had a significant influence on the gold dissolution behaviors. It can be seen from Figure 5 that the open circuit potential of gold first shifted toward the cathode direction and then toward the anodic direction with increasing the pH. At the same time, when pH was increased from 10.5 to 11.0, the polarization curves of gold rose, whereas when the increase of pH from 11.0 to 12.0 moved down the polarization curves dropped. At different solution pH values, a weak current peak there occurred on the gold anode polarization curves. When the pH was increased from 10.5 to 12.0, the peak potential shifted toward the cathode direction and the peak current density reduced. This indicated that in the presence of chalcopyrite, the optimal pH range for dissolution of gold was 10.5 to 11.0 and that in the presence of chalcopyrite, the proper increase of solution pH was conducive to accelerate the dissolution rate of gold. However, at high pH values (beyond 11.0), the anode dissolution rate of gold was lowered, which could be due to the presence of more hydroxides that promoted the formation of passivating gold surface products. This phenomenon is similar to the effect of pH on the pure gold electrode in clean cyanide solution [34].

Figure 5 Effect of pH on gold anodic polarization curves (Conditions: scan rate=1.0 mV/s, ρ(NaCN)=700 mg/L, T=298 K, disc rotation speed=400 r/min, DO=0)
3.4 Effect of rotation rate
To study the influence of diffusion on the dissolution rate of gold in the presence of chalcopyrite, the polarization curves of gold at different rotation rates were measured (see Figure 6). The results showed that the rotation rate had a significant influence on the dissolution of gold. The gold anode polarization curves showed a weak current peak near -130 mV and the peak slightly shifted with increasing rotation rate, but the peak current density increased remarkably. Simultaneously, the effect of electrode speed on the anode dissolution behavior of gold was significantly different at the varied potentials, so the gold anode polarization curve was divided into two regions as A (from the open circuit potential to 0 mV) and B (from 0 mV to 166 mV). In region A, the peak current density increased by about 1.1 times (from 4.10 A/m2 to 8.51 A/m2) when the electrode speed increased from 0 to 200 r/min, but only by about 35.6% (from 8.51 A/m2 to 11.54 A/m2) when it increased from 200 to 600 r/min. However, when the electrode speed was increased from 600 to 800 r/min, the peak current density was decreased by around 20.36% (from 11.54 A/m2 to 9.17 A/m2). In region B, as the electrode speed was increased from 0 to 600 r/min, the anode current density continuously increased, but the increasing degree weakened with increasing electrode speeds. However, when the electrode speed increased from 600 to 800 r/min, the anode current density of gold sharply increased again. From the figure, it could be seen that in region A, the anode current density of gold was lower at 800 r/min than that at 600 r/min while in region B, the anode current density of gold was higher at 800 r/min than that at 600 r/min. Repeated tests obtained the same results.

Figure 6 Effect of disc rotation speed on gold anodic polarization curves (1-0; 2-200 r/min; 3-400 r/min; 4-600 r/min; 5-800 r/min in conditions: scan rate=1.0 mV/s, ρ(NaCN)=700 mg/L, pH=10.5, T=298 K, DO=0)
The analysis indicated that in the presence of chalcopyrite, the effect of the electrode speeds on the dissolution rate of gold was complicated at low potential region (in region A) but simplified at high potential (in region B), in which diffusion had a significant influence on the dissolution rate of gold.
3.5 Effect of temperature
Figure 7 shows the anodic polarization curve of gold at different temperatures in the presence of chalcopyrite. As shown in Figure 7, temperature has a significant influence on the dissolution of gold in a cyanide solution. With temperature increasing, the open circuit potential of gold remarkably shifted toward the cathode direction; the peak current density significantly increased, and the peak potential was slightly moved toward the negative potential direction. This indicated that increasing the temperature could reduce the open circuit potential of gold and significantly increase the dissolution rate of gold. The theoretical formula of activation energy in electrochemical systems is as follows:
 (3)
(3)
where J is the current density; η is the overpotential; E is the activation energy; T is the temperature (K); R is the gas constant equal to 8.314 J/(mol·K) and B is constant. The logarithm of both sides of Eq. (3) was taken to get the following formula:
 (4)
(4)
According to Eq. (4), the current density at-6 mV was selected from Figure 7 to calculate the activation energy of dissolution of gold (see Figure 8), and the calculation result was 31.20 kJ/mol. According to the above calculation method, the relationship between over-potential and activation energy was obtained with the data in Figure 7 (see Figure 9). As shown in Figure 9, the activation energy decreased with the positive increase of over-potential. Therefore, the minimum activation energy of the dissolution of gold in the negative potential region was 31.20 kJ/mol. However, when the over-potential increased from -6 mV to 166 mV, the activation energy was lowered from 31.20 kJ/mol to 16.74 kJ/mol. This indicated that in the presence of chalcopyrite, the dissolution rate of gold was controlled by electrochemical reaction in the low potential region while in the high region, the control steps of gold anodic dissolution rate changed from electrochemical reaction control to diffusion control or mixed control.

Figure 7 Effect of temperature on gold anodic polarization (Curves: 1-293 K; 2-303 K; 3-308 K; 4-313 K under conditions: scan rate=1.0 mV/s, ρ(NaCN)=700 mg/L, pH=10.5, disc rotation speed=600 r/min, DO=0)
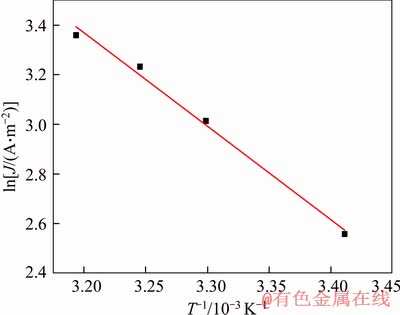
Figure 8 Relationship curve of lnJ vs T-1 at E=-6 mV

Figure 9 Electrode reaction activation energy changing with electrode potential
4 Conclusions
The dissolution of pure gold was studied in de-aerated cyanide solutions containing chalcopyrite. From the results presented in this paper, the following conclusions could be drawn:
1) The results obtained in this work indicate that the presence of small amounts of chalcopyrite to de-aerated cyanide solutions would significantly decrease the anodic active dissolution rate of gold at lower potentials, but it would remarkably increase the anodic activity of gold at more positive potentials, thus accelerating the dissolution of gold.
2) At low potential, the effect of cyanide concentration on gold anodic dissolution rate is complex. When the cyanide concentration was below 980 mg/L, the dissolution of gold in the de-aerated cyanide solutions was controlled by cyanide diffusion; when the cyanide concentration was beyond 980 mg/L, the dissolution of gold in the de-aerated cyanide solutions was controlled by chemical reaction. However, at higher potential, dissolution of gold is only controlled by cyanide diffusion.
3) With increasing temperature, the open circuit potential of gold lowers and the dissolution of gold significantly improves. In the presence of chalcopyrite, appropriately increasing the pH value of the solution is conducive to the anodic dissolution of gold. However, too high pH value will reduce the current density of dissolution and promote the generation of passivation, which is not conducive to the cyanide dissolution of gold.
References
[1] BAS A D, GHALI E, CHOI Y. A review on electrochemical dissolution and passivation of gold during cyanidation in presence of sulphides and oxides [J]. Hydrometallurgy, 2017, 172: 30-44. DOI: 10.1016/j.hydromet.2017.12.016.
[2] KUDRYK V, KELLOGG H H. Mechanism and rate-controlling factors in the dissolution of gold in cyanide solution [J]. JOM, 1954, 6(5): 541-548. DOI: 10.1007/ BF03398872.
[3] JEFFREY M I, RITCHIE I M. The leaching and electrochemistry of gold in high purity cyanide solutions [J]. Journal of the Electrochemical Society, 2001, 148(4): D29-D36. DOI:10.1149/1.1353573.
[4] MRKUSIC D, PAYNTER J. The recovery of gold from sulphidic and arsenical ores mainly from the Barberton area [R]. Johannesburg: National Institute for Metallurgy, 1970: 911.
[5] GUAN Y, HAN K N. An electrochemical study on the dissolution of gold and copper from gold/copper alloys [J]. Metallurgical & Materials Transactions B, 1994, 25(6): 817-827. DOI: 10.1007/BF02662764.
[6] YANG Bao-jun, ZHAO Chun-xiao, LUO Wen, LIAO Rui, GAN Min, WANG Jun, LIU Xue-duan, QIU Guan-zhou. Catalytic effect of silver on copper release from chalcopyrite mediated by Acidithiobacillus ferrooxidans [J]. Journal of Hazardous Materials, 2020, 392: 122290. DOI: 10.1016/ j.jhazmat. 2020.122290.
[7] YANG Bao-jun, Lin Mo, Fang Jing-hua, Zhang Rui-yong, Luo Wen, Wang Xing-xing, Liao Rui, Wu Bai-qiang, Wang Jun, Gan Min. Combined effects of jarosite and visible light on chalcopyrite dissolution mediated by Acidithiobacillus ferrooxidans [J]. Science of the Total Environment, 2020, 698: 134175. DOI: 10.1016/ j.scitotenv.2019.134175.
[8] TSHILOMBO A F, SANDENBERGH R F. Electrochemical study of the effect of lead and sulphide ions on the dissolution rate of gold in alkaline cyanide solutions [J]. Hydrometallurgy, 2001, 60(1): 55-67. DOI: 10.1016/S0304- 386X(00)00157-2.
[9] BAS A D, KOC E, YAZICI E Y, DEVECI H. Treatment of copper-rich gold ore by cyanide leaching, ammonia pretreatment and ammoniacal cyanide leaching [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(2): 597-607. DOI: 10.1016/S1003-6326(15)63642-1.
[10] DESCHENES G, PRUD'HOMME P J H. Cyanidation of a copper-gold ore [J]. International Journal of Mineral Processing, 1997, 50: 127-141. DOI: 10.1016/S0301- 7516(97)00008-2.
[11] Lan Zhuo-yue, HU Yue-hua, Liu Jian-she, Wang Jun. Solvent extraction of copper and zinc from bioleaching solutions with LIX984 and D2EHPA [J]. Journal of Central South University of Technology, 2005, 12(1): 45-49. DOI: 10.1007/s11771-005-0201-z.
[12] Wang Jun, Qin Wen-qing, Zhang Yan-sheng, Yang Cong-ren, Zhang Jian-wen, Lai Shao-shi, Shang He, Qiu Guan-zhou. Bacterial leaching of chalcopyrite and bornite with native bioleaching microorganism [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1468-1472. DOI: 10.1016/S1003-6326(09)60027-3.
[13] Anderson C G. Alkaline sulfide gold leaching kinetics [J]. Minerals Engineering, 2016, 92: 248-256. DOI: 10.1016/j.mineng.2016.01.009.
[14] Azizia A, Petreb C F, Olsenb C, lLarachia F. Electrochemical behavior of gold cyanidation in the presence of a sulfide-rich industrial ore versus its major constitutive sulfide minerals [J]. Hydrometallurgy, 2010, 101(3, 4): 108-119. DOI: 10.1016/j.hydromet.2009.12.004.
[15] DESCHENES G, GUO H, XIA C, PRATT A, FULTON M, CHOI Y, PRICE J. A study of the effect of djurliete, bornite and chalcopyrite during the dissolution of gold with a solution of ammonia-cyanide [J]. Minerals, 2012, 2(4): 459-472. DOI: 10.3390/min2040459.
[16] FISHER W W. Comparison of chalcocite dissolution in the sulfate, perchlorate, nitrate, chloride, ammonia, and cyanide systems [J]. Minerals Engineering, 1994, 7(1): 99-103. DOI: 10.1016/0892-6875(94)90150-3.
[17] LA BROOY S R, LINGE H G, WALKER G S. Review of gold extraction from ores [J]. Minerals Engineering, 1994, 7(10): 1213-1241. DOI: 10.1016/0892-6875(94)90114-7.
[18] BRICENO A, CHANDER S. Oxidation of hydrosulphide ions on gold, Part I: A cyclic voltammetry study [J]. Journal of Applied Electrochemistry, 1990, 20(3): 506-511. DOI: 10.1007/BF01076064.
[19] HAMILTON I C, WOODS R. An investigation of the deposition and reactions of sulphur on gold electrodes [J]. Journal of Applied Electrochemistry, 1983, 13(6): 783-794. DOI: 10.1007/bf00615828.
[20] JEFFREY M I, BREUER P L. The cyanide leaching of gold in solutions containing sulfide [J]. Minerals Engineering, 2000, 13(10): 1097-1106. DOI: 10.1016/S0892-6875(00) 00093-5.
[21] LORENZEN L, DEVENTER J S J V. Electrochemical interactions between gold and its associated minerals during cyanidation [J]. Hydrometallurgy, 1992, 30(1-3): 177-193. DOI: 10.1016/0304-386X(92)90083-C.
[22] Zhao Hong-bo, Wang Jun, Gan Xiao-wen, Hu Ming-hao, Tao Lang, Qin Wen-qing, Qiu Guan-zhou. Role of pyrite in sulfuric acid leaching of chalcopyrite: An elimination of polysulfide by controlling redox potential [J]. Hydrometallurgy, 2016, 164: 159-165. DOI: 10.1016/ j.hydromet.2016.04.013.
[23] AGHAMIRIAN M M, YEN W T. Mechanisms of galvanic interactions between gold and sulfide minerals in cyanide solution [J]. Minerals Engineering, 2005, 18(4): 393-407. DOI: 10.1016/j.mineng.2004.07.005.
[24] WANG Jun, ZHOU Hong-bo, QIN Wen-qing, QIU Guan-zhou. Bioleaching of complex polymetallic sulfide ores by mixed culture [J]. Journal of Central South University, 2014, 21(7): 2633-2637. DOI: 10.1007/s11771- 014-2223-x.
[25] HUANG X, ZHAO H B, ZHANG Y, LIAO R, WANG J, QIN W Q, QIU G Z. A strategy to accelerate the bioleaching of chalcopyrite through the goethite process [J]. Minerals & Metallurgical Processing, 2018, 35(4): 171-175. DOI: 10.19150/mmp.8593.
[26] Zhao Hong-bo, Gan Xiao-wen, Wang Jun, Tao Lang, Qin Wen-qing, Qiu Guan-zhou. Stepwise bioleaching of Cu-Zn mixed ores with comprehensive utilization of silver-bearing solid waste through a new technique process [J]. Hydrometallurgy, 2017, 171: 374-386. DOI: 10.1016/ j.hydromet.2017.06.002.
[27] Wang Jun, Liao Rui, Tao Lang, Zhao Hong-bo, Zhai Rui, Qin Wen-qing, Qiu Guan-zhou. A comprehensive utilization of silver-bearing solid wastes in chalcopyrite bioleaching [J]. Hydrometallurgy, 2017, 169: 152-157. DOI: 10.1016/j.hydromet.2017.01.006.
[28] Fang Jing-hua, Liu Yong, He Wan-li, Qin Wen-qing, Qiu Guan-zhou, Wang Jun. Transformation of iron in pure culture process of extremely acidophilic microorganisms [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(5): 1150-1155. DOI: 10.1016/S1003-6326(17)60134-1.
[29] ZHao Hong-bo, Wang Jun, Gan Xiao-wen, Hu Ming-hao, Zhang Er-xing, Qin Wen-qing, Qiu Guan- zhou. Cooperative bioleaching of chalcopyrite and silver- bearing tailing by mixed moderately thermophilic culture: An emphasis on the chalcopyrite dissolution with XPS and electrochemical analysis [J]. Minerals Engineering, 2015, 81: 29-39. DOI: 10.1016/j.mineng.2015.07.015.
[30] Wang Jun, QIU Guan-zhou, QIN Wen-qing, ZHANG Yan-sheng. Microbial leaching of marmatite by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(4): 937-942. DOI: 10.1016/s1003- 6326(06)60355-5.
[31] BEK R Y, SHURAEVA L I. The effect of sulfide ion chemisorption on the kinetics of gold dissolution in cyanide solutions [J]. Russian Journal of Electrochemistry, 2008, 44(7): 767-771. DOI: 10.1134/s102319350807001x.
[32] ZHENG J, RITCHIE I M, BROOY S R LA, SINGH P. Study of gold leaching in oxygenated solutions containing cyanide-copper-ammonia using a rotating quartz crystal microbalance [J]. Hydrometallurgy, 1995, 39(1-3): 277-292. DOI: 10.1016/0304-386X(95)00036-G.
[33] Yang Yong-bin. Investigation on electrochemical kinetics and application for co-investigation of gold leaching [D]. Changsha: School of Minerals Processing and Bioengineering, Central South University, 2008. (in Chinese)
[34] LIN H K, CHEN X. Electrochemical study of gold dissolution in cyanide solution [J]. Mining, Metallurgy & Exploration, 2001, 18(3): 147-153. DOI: 10.1007/ BF03402888
(Edited by YANG Hua)
中文导读
金在含黄铜矿的氰化物溶液中的溶解
摘要:利用旋转圆盘电极研究了金在有黄铜矿存在的缺氧氰化物溶液中的阳极溶解特性。研究发现:黄铜矿的存在会削弱金在低电位区的氰化溶解活性,但会增强其在高电位区的溶解活性。在电位≤177 mV时,金的溶解过程控制步骤与体系中氰化物浓度有关,当氰化物浓度低于980 mg/L时,金的溶解过程受扩散控制;当氰化物浓度高于980 mg/L时,金的阳极溶解电流密度降低,溶解过程由扩散控制转变为电化学反应控制。在电位>177 mV时,金的阳极溶解始终受扩散控制,而与体系中氰化钠浓度无关。同时,在含黄铜矿的氰化物溶液中,适当提高体系pH值和升高温度可以促进金阳极溶解,但是pH>11.5也会促进钝化作用的产生而对金的氰化溶解不利。
关键词:黄铜矿;氰化物;金;溶解
Foundation item: Project(51474169) supported by the National Natural Science Foundation of China; Project(18JS061) supported by the Key Laboratory Research Project of Education Department in Shaanxi Province, China
Received date: 2019-06-21; Accepted date: 2020-04-30
Corresponding author: YANG Wei, PhD, Professor; Tel: +86-15009267016; E-mail: yangweixauat@126.com; ORCID: 0000-0001- 6390-3141

