Article ID: 1003-6326(2005)03-0589-05
Kinetic equation based on non-steady-state
diffusion of oxygen in solid copper
SONG Ke-xing(宋克兴)1, 2, GAO Jian-xin(郜建新)2, JIANG Xi-xiang(姜细想)3,
CHEN Yi-bin(陈益斌)3, XING Jian-dong(邢建东)1
(1. State Key Laboratory for Mechanical Behavior of Materials,
Xian Jiaotong University, Xian 710049, China;
2. School of Materials Science and Engineering,
Henan University of Science and Technology, Luoyang 471003 , China;
3. Dongfeng Motor Co Ltd, Shiyan 442042, China)
Abstract: The kinetics of internal oxidation of dilute Cu-Al alloys, containing 0.4475%-2.214%Al (mole fraction) was investigated over the temperature range of 1023-1273K and the depth of internal oxidation was measured by microscopy. Based on non-steady-state diffusion, a rate equation is derived to describe the kinetics of internal oxidation of plate: X=k[KF(]t[KF)], where X is the oxidation depth, t is the oxidation time. For the internal oxidation of Cu-Al alloys employed in the synthesis of alumina dispersion strengthened copper, the permeability of oxygen in solid copper is obtained from the internal oxidation measurements. Investigation shows that the depth of the internal oxidation is a parabolic function of time, the typical shape of the front of internal oxidation is of planar morphology, and there is no evidence for preferential diffusion along grain boundaries.
Key words: Cu-Al alloys; non-steady-state diffusion; internal oxidation; rate equation CLC
number: TG146.1 Document code: A
1 INTRODUCTION
Internal oxidation provides a method of dispersion hardening. Considerable efforts have been devoted to this method and encouraging results have been obtained in some systems, for example, Cu-Al, Cu-Cr, Cu-Ti, and Co-Ti alloys[1-5]. And now internal oxidation has become the most convenient method for producing oxide dispersion strengthened (ODS) copper on a commercial scale[6-11].
The internal oxidation rate equation based on quasi-steady-state assumption is often referred in the literatures[5, 12-14]. On the other hand, the non-steady-state diffusion rate equation is seldom reported. The diffusion of oxygen in solid copper during internal oxidation is considered in the non-steady-state, and the research on non-steady-state diffusion rate equation will be necessary in order to better describe the kinetics of internal oxidation.
In the present research, the non-steady-state diffusion rate equation for internal oxidation of Cu-Al alloy plate was derived. According to this equation, the permeability of oxygen in solid copper was estimated from the internal oxidation measurements in Cu-Al alloys.
2 EXPERIMENTAL
The Cu-Al alloys for internal oxidation were prepared by melting oxygen-free high conductivity copper (≥99.95%) together with electrolytic aluminum (≥99.90%) in a vacuum induction furnace. The melts were cast into ingots of 80mm in diameter and 150mm in length. The aluminum mole fractions (xAl) of the prepared Cu-Al alloy ingots are 0.4475%, 0.9892%, 1.6957%, and 2.2140%, respectively. Then the ingots were scalped and swaged to square bar of 14mm×14mm. The square bars were then annealed for homogenization at 1173K in argon for 10h. The oxidation samples of 120mm×10mm×1.5mm were cut from the as-annealed bars. In order to standardize the surface condition, each sample was cleaned and smoothed on fine metallographic emery paper prior to the oxidizing treatment.
In order to induce internal oxidation without formation of a surface scale, the specimens were packed in a powder mixture of 30%Cu2O, 20%Cu and 50%Al2O3, and sealed in evacuated copper containers. The Cu and Cu2O powders were served to establish the oxygen partial pressure at the dissociation pressure of Cu2O while the Al2O3 was served to minimize sintering of the mixture. Each container held one specimen of each alloy. Oxidation temperatures of 1023, 1123, 1223, and 1273K were used with at least four oxidation times being employed for each temperature.
The oxidized planar specimens were mounted and sectioned perpendicular to the broad face with at least 2mm being removed from the length in order to eliminate end effects. After metallographic preparation, the depth of oxidation of each sample was measured at 10 points in the cross section under an optical microscope equipped with a micro-meter stage. The OM and SEM micrographs were taken to the samples.
3 RESULTS AND CALCULATION
3.1 Profile of interface
Fig.1 shows that the typical shape of internal oxidation front is of planar morphology, and there is no evidence for preferential diffusion along grain boundaries. This suggests that in the process of internal oxidation, oxygen permeates mainly in the form of volume diffusion.
3.2 Kinetic equation
Internal oxidation is a process by which oxygen diffuses into an alloy and causes sub-surface precipitation of the oxides of one or more alloying elements.
Considering a planar specimen of Cu-Al alloy in which Al is a dilute solute and forms a very stable oxide (Al2O3), as a simplification to internal oxidation kinetics, the following approximations have been made(Fig.2).
(a) (DAl=0): the diffusion coefficient of solute Al in Cu (DAl) can be neglected, which means that the counter-diffusion of solute Al is assumed to be negligible.
(b) (cO=constant): the oxygen is taken up rapidly from the oxidizing atmosphere, so that the surface oxygen concentration is practically equal to the solubility (cO) of oxygen in Cu.
(c) (Sharp moving boundary): the reaction takes place only and completely at a sharp boundary plane between the internally oxidized and the remaining part of the specimen. This means in front of the advancing sharp boundary, the concentration of freely diffusing oxygen is zero, while behind it all of the solute Al in Cu-Al alloys has been converted to Al2O3.
Suppose the oxidation front (moving boundary) at time t is at point of X=X(t).
According to Ficks second law, in the region 0〈x〈X, there is
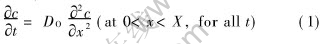
where c is the oxygen concentration.
And c=cO (at x=0 for all t)(2)
At the moving boundary:
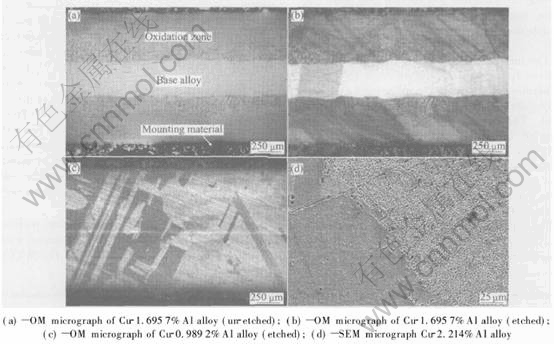
Fig.1 Photomicrographs of Cu-Al alloys after partial internal oxidation for 10h at 1273K
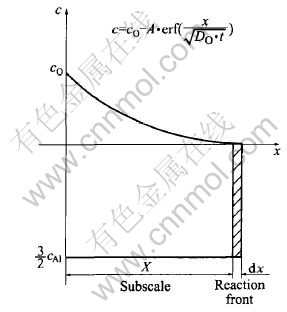
Fig.2 Schematic concentration profiles of internal oxidation
c=0 (x=X for all t)(3)
In order that the reaction front can advance a distance δX, we need to supply an amount (3/2)cAlδX of oxygen. According to Ficks first law, the amount of oxygen arriving at X in a time interval δt is -DOδtc/xx=X, and conservation at boundary requires:
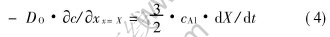
The solution of equation (1) satisfying equation (2) is
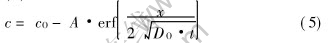
where A is a constant, erf(·) is the error function. Erf 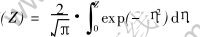
The constant A is determined by the condition (3), which gives

Since equation (6) has to be satisfied for all values of t, 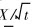 must be a constant, that is
must be a constant, that is
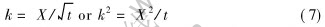
Considering boundary condition (4), we find
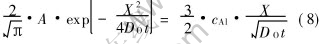
Substitution of equation (7) into equation (6) and equation (8) yields

Eliminating A from equations (9) and (10), we obtain
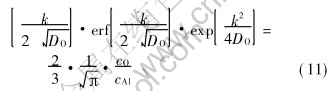
Here the rate equation to describe the kinetics of internal oxidation is  . The value of the constant k is determined by equation (11).
. The value of the constant k is determined by equation (11).
The relation between the ratio cO/cAl and 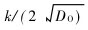 is represented in Fig.3.
is represented in Fig.3.
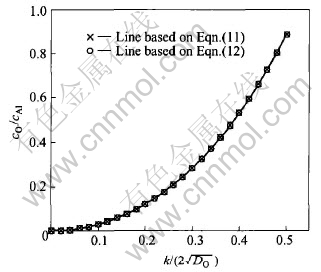
Fig.3 Relation between cO/cAl and 
For the internal oxidation of Cu-Al alloys employed in the synthesis of alumina dispersion strengthened copper, the value of ratio cO/cAl is small (for example, at 1323K, cO=2.66×10-4 according to Pastorek[15], when aluminum content is 0.1%(mass fraction), cAl=2.3553×10-3, then the value of ratio cO/cAl is only 0.113161).
Considering the condition of k/(  ≤0.5(cO/cAl≤0.9), the equation (11) describing the relation between the ratio cO/cAl and
≤0.5(cO/cAl≤0.9), the equation (11) describing the relation between the ratio cO/cAl and  can be substituted by (as shown in Fig.3)
can be substituted by (as shown in Fig.3)
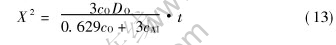
By substituting equation (12) into equation (7), we obtain

3.3 Check and calculation
The measured data of internal oxidation depth are summarized in Fig.4. It shows that the relation between oxidation depth (X) and time (t) is in good agreement with the result from equation (7). The k2 values calculated according to equation (7) are given in Fig.4 as a plot of square of thickness, X2 vs time, cm2/s.
Fig.5 shows the variation of k2 values (ob- tained from Fig.4) with cAl at different tempera-
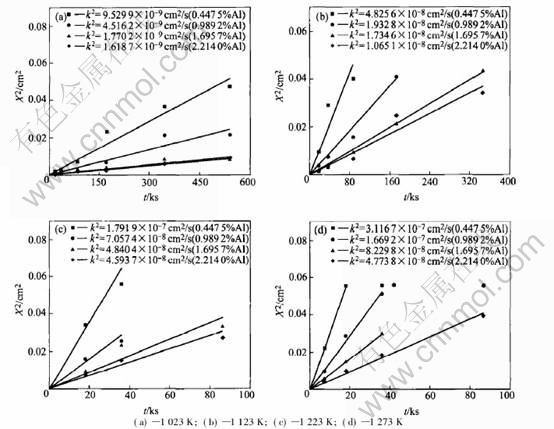
Fig.4 Variations of oxidation depth with time at different temperatures
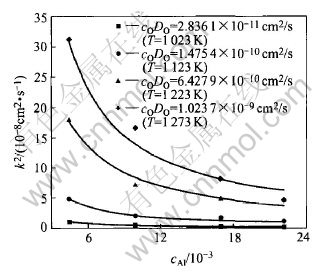
Fig.5 Variation of k2 with cAl at different temperatures
tures. By fitting the data of k2 with equation (12), the values of cODO and cO can be calculated. However, the calculated data of cO are ineffective, and this is because the values of cO are so sensitive to k2 that the mini error of k2 (or X2 obtained from the measurements of internal oxidation) will lead to the large error of cO. So here we can only get the effective data of cODO, as shown in Fig.5.
From the calculated data of cODO against 1/T shown in Fig.6, we can get the permeability of oxygen in solid copper:
cODO=1.95×10-2exp(-41711/RT)(14)
The energy Q associated with temperature de- pendence of cODO is 175.14kJ according to equation (14).
Fig.6 Permeability cODO(cm2/s) of oxygen in solid copper
4 DISCUSSION
From the above study, we can see: (a) the relation between internal oxidation depth (X) and time (t) is in good agreement with the result from the derived equation (7); (b) the energy Q associated with temperature dependence of cODO calculated from the measured data and the derived equation (12) is 175.14kJ, which is in agreement with the value of 165.90kJ obtained by Meijering[16] and somewhat lower than the value of 190.04kJ obtained by Pastorek[15] from electrochemical measurements. This means that the derived equation based on non-steady-state diffusion of oxygen in solid copper can better describe the kinetics of internal oxidation of Cu-Al alloy plates.
With the development of synthesis technology of alumina dispersion strengthened copper, the researches on internal oxidation kinetics based on no-steady-state diffusion will be important: (a) the process of internal oxidation is diffusion controlled, and the diffusion of oxygen in solid copper is in non-steady-state; (b) these studies can provide basic knowledge for optimizing internal oxidation parameters during the synthesis of alumina dispersion strengthened copper plates, and for obtaining fine oxide particles which play a decisive role in the high temperature properties of alumina dispersion strengthened copper.
5 CONCLUSIONS
1) The kinetic equation of internal oxidation of Cu-Al alloy plate based on non-steady-sate diffusion can be described as 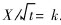 . And the value of constant k is determined by
. And the value of constant k is determined by

For the internal oxidation of Cu-Al alloys employed in the synthesis of alumina dispersion strengthened copper, the rate equation can be simplified as
X2=4cODOt/(0.629cO+3cAl)
2) The permeability of oxygen in solid copper is cODO=1.95×10-2exp(-41711/RT)
3) The typical shape of internal oxidation front is of planar morphology, and there is no evidence for preferential diffusion along grain boundaries.
REFERENCES
[1]SONG Ke-xing, XING Jian-dong, DONG Qi-ming, et al. Internal oxidation of dilute Cu-Al alloy powders with oxidant of Cu2O[J]. Materials Science and Engineering, 2004, A380: 117-122.
[2]Groza J R, Gibeling J C. Principle of particle section for dispersion-strengthened copper[J]. Materials Science and Engineering, 1993, A171: 115-125.
[3]ZHOU Guo-hong, LI Hua-lun, HU Rui. On better understanding of dispersion-separation mechanism in internal oxidation of aluminum in Cu-Al alloy[J]. Journal of Northwestern Polytechnical University, 2002, 20(2): 176-179. (in Chinese)
[4]LIANG Shu-hua, FANG Liang, FAN Zhi-kang. Internal oxidation of Cr in Cu-Cr/Cu2O composite powder prepared by mechanical activation[J]. Materials Science and Engineering, 2004, A374: 27-33.
[5]Nadkarni A V. Dispersion strengthened copper properties and applications [A]. Ling E, Taubenblat P W. High Conductivity Copper and Aluminum Alloys[C]. Warrendale: Metall Soc AIME, 1984. 77 - 101.
[6]WU Jian-jun, ZHANG Yun, SHEN Yu-tian, et al. Internal oxidation of Cu-Al alloy[J]. J Mater Sci Technol, 1999, 15(5): 444-448.
[7]SHI Zi-yuan, YAN Mao-fang. The preparation of Al2O3-Cu composite by internal oxidation[J]. Applied Surface Science, 1998, 134: 103-106.
[8]Srivatsan T S, Narendra N, Troxell J D. Tensile deformation and fracture behavior of an oxide dispersion strengthened copper alloy[J]. Materials and Design, 2000, 21: 191 - 198.
[9]CHENG Jian-yi, WANG Ming-pu, LI Zhou, et al. Fabrication and properties of low oxygen grade Al2O3 dispersion strengthened copper alloy[J]. Trans Nonferrous Met Soc China, 2004, 14(1): 121-126.
[10]CHENG Jian-yi, WANG Min-pu, ZHONG Wei-jia, et al. Properties and microstructures of Cu- Al2O3 alloy produced by internal oxidation[J]. Transactions of Materials and Heat Treatment, 2003, 24(1): 23 - 27. (in Chinese)
[11]SONG Ke-xing,XING Jian-dong, TIAN Bao-hong, et al. Influence of annealing treatment on the properties and microstructures of alumina dispersion strengthened copper alloy[J]. Trans Nonferrous Met Soc China, 2005, 15(1): 1-6.
[12]Rhines F N, Johnson W A, Anderson W A. Rates of high-temperature oxidation of dilute copper alloys[J]. Transactions of the Metallurgical Society of AIME, 1942, 147: 205-221.
[13]Birks N, Meier G H. Oxidation of Metals and Alloys[M]. London: Edward Arnold Ltd, 1983. 235-267.
[14]Tian S G, Zhang L T, Shao H M, et al. Internal oxidation kinetics & diffusion mechanism of oxygen in Cu-Al sintered alloy[J]. Acta Metallurgica Sinica, 1996, 9(5): 387-390.
[15]Pastorek R L, Rapp R A. The solubility and diffusivity of oxygen in solid copper from electrochemical measurements[J]. Transactions of the Metallurgical Society of AIME, 1969, 245: 1711-1720.
[16]Merjering J L. Internal oxidation in alloys [A]. Herman H. Advances in Materials Research[C]. New York: Wilkey-Interscience, 1971. 1-81.
(Edited by YANG Bing)
Foundation item: Project (2002AA331112) supported by Hi-tech and Development Program of China; Project (0122021300) supported by the Natural Science Foundation of Henan Province, China
Received date: 2004-12-10; Accepted date: 2005-03-08
Correspondence: SONG Ke-xing, Associate Professor, PhD; Tel: +86-379-64231892; Fax: +86-379-64230597; E-mail: kxsong@mail.haust.edu.cn