
Fabrication and characteristics of rutile TiO2 nanoparticles induced by laser ablation
LIU Pei-sheng(刘培生)1,2,3,4,CAI Wei-ping(蔡伟平)2,WAN Li-xi(万里兮)3,
SHI Ming-da(石明达)4, LUO Xiang-dong(罗向东) 1, JING Wei-ping(景为平)1
1. Jiangsu Key Laboratory of ASCI Design, Nantong University, Nantong 226019, China;
2. Key Laboratory of Materials Physics, Anhui Key Laboratory of Nanomaterials and Nanotechnology,
Institute of Solid State Physics, Chinese Academy of Sciences, Hefei 230031, China;
3. Institute of Microelectronics, Chinese Academy of Sciences, Bejing 100029, China;
4. Nantong Fujitsu Microelectronics Co. Ltd, Nantong 226006, China
Received 10 August 2009; accepted 15 September 2009
Abstract: The laser ablation technique was employed to prepare TiO2 nanoparticles by pulsed laser ablation of a titanium target immersed in the poly-(vinylpyrrolidone) solution using wavelength of 1 064 nm. The as-prepared products were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The results indicate that the rutile TiO2 nanoparticles are synthesized at room temperature and the average size is about 35 nm with narrow size distribution. A possible formation mechanism was discussed and the UV-vis absorption and photoluminescence were measured. The optical study shows that rutile nanoparticle possesses direct optical transition with band gap of 3.15 eV.
Key words: TiO2 rutile; laser ablation; nanoparticles; photoluminescence
1 Introduction
TiO2 has been investigated for many years. Titanium dioxide is a fascinating class of inorganic solids in a wide range of common and high technique applications due to its wide application in the photocatalysis, optical materials, dye-sensitized solar cell, lithium-ion batteries fields[1-4]. Crystalline TiO2 exists in three forms: rutile, anatase and brookite, of which, rutile is the most thermodynamically stable phase (generally at 600-1 855 ℃), whereas anatase and brookite phases are metastable and readily transformed to rutile phase when heated. Rutile TiO2 has some advantages over anatase phase, such as higher refractive index, higher dielectric constant, higher electric resistance and higher chemical stability. Rutile TiO2 has been traditionally used in pigment, plastic, construction and cosmetic fields because of its good light-scattering and light-reflecting effect, nontoxicity and chemical inertness. In the electronics industry, the rutile TiO2 is used because of the high dielectric constant and high electrical resistance, and also used in capacitor, filter, power circuits and temperature compensating condensers [5]. These properties will be superior if the titania has high surface area.
Rutile TiO2 nanoparticles are usually synthesized by calcinating anatase TiO2 nanoparticles or amorphous TiO2 at higher temperature more than 450℃. Two-step process is needed and high-temperature calcination unavoidably leads to the agglomeration and growth of nanocrystallites. Hydrolysis of TiCl4 in aqueous solution can form rutile nanocrystals at relatively low temperatures[6-7]. However, this method is easily affected by the pH value and autoclaving temperature and cannot be controlled easily. Therefore, the preparation of rutile nanocrystals by one-step method at room temperatures would be significant.
In recent years, pulsed laser ablation of metal target in liquid media has attracted great interest because such laser ablation in liquid (LAL) can produce the extreme conditions and lead to the formation of the novel nanostructures [8-10]. When a pulsed laser beam with enough energy irradiates on a metal target in a
transparent liquid, a local plasma with super-high temperature (about 6 000 K) and high-pressure (about 1 GPa) will instantly be produced on the solid-liquid interface, and quench quickly after one pulse due to adiabatic expansion of the plasma and its interaction with surrounding media. The whole process is finished in about 1 μs. So, the laser ablation of metal targets in liquid media can form some special nanomaterials that are difficult to be obtained by the conventional methods.
In this work, we reported successful one-step fabrication of rutile TiO2 nanoparticles by LAL of titanium plate in poly-(vinylpyrrolidone) (PVP) solution at room temperature.
2 Experimental
Titanium plate with thickness of 2 mm, diameter of 15 mm and higher than 99.9% purity was used. The plate was first fixed on a bracket in a quartz glass vessel filled with 10 ml 0.01 mol/L PVP (K-30, Mw=40 000, Aldrich) aqueous solution, which was continuously stirred. The plate was kept at about 4 mm below the solution surface. The laser used in the experiments (λ=1 064 nm) was set at energy of 80 mJ per pulse and the pulse width of 10 ns. The beam was collimated to ensure homogeneous energy density and focused to spot size using quarz lens with focal length of 250 mm, which corresponded to the incident power density required by the experiments. The energy density was estimated from the supplied laser energy and the spot size. The laser energy was measured with laser power meter (OPHIR). The experimental equipment is shown in Fig.1.
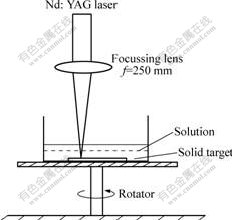
Fig.1 Schematic diagram of experimental equipment for liquid laser ablation
The titanium plate was irradiated for 60 min by the pulse laser. After irradiation, the solutions were centrifuged at 14 000 r/min. The obtained powder- products were ultrasonically rinsed with ethanol for six times to remove the surfactant molecules on the particles as much as possible, and then draught-dried at room temperature.
X-ray diffraction (XRD) (Philips X’Pert using Cu Kα line 0.154 19 nm) was used to analyze these powder samples. For transmission electron microscopic (TEM) examination, the powder samples were ultrasonically re-dispersed in ethanol, before it was dropped on the copper grids coated with thin carbon film and evaporated in air at room temperature. TEM observation was conducted on a JEOL 2010 TEM, operating at an accelerating voltage of 200 kV. X-ray photoelectron spectra (XPS) were collected by an ESCALab MKII X-ray photoelectron spectrometer using nonmono- chromatized MgKα X-ray as the excitation source. The optical absorption spectra of the irradiated solutions were measured immediately on a Cary 5E UV-vis-NIR spectrophotometer using an optical quartz cell with path length of 10 mm. The wavelength varies from 200 to 800 nm. The photo luminescence(PL) spectra were measured by an Edinburgh luminescence spectrometer (FLS 920) with Xe lamp excitation at room temperatures.
3 Results and discussion
3.1 Structure and morphology
Fig.2 shows the XRD pattern of the products prepared in 0.01 mol/L PVP solution. It can be seen from Fig.2 that the XRD pattern is in agreement with standard diffraction of rutile TiO2 powders (JCPDS file No. 77-0441).

Fig.2 XRD pattern of as-prepared sample
The morphologies of particles were observed by TEM. From Fig.3, the products are nearly spherical shape. There are little aggregations observed in the as-prepared sample. The particle size distribution is measured from Fig.3. Fig.4 shows the particle size distribution histogram for TiO2 nanoparticles. It is seen from the histogram (Fig.4) that the diameter of particles ranges between 18 and 50 nm and the average size of particles is 35 nm with a standard deviation of 12, which have narrow size distribution.

Fig.3 TEM image of samples prepared in 0.01 mol/L PVP solution

Fig.4 Particle size distribution of sample
3.2 XPS analyses
Fig.5 shows the XPS spectrum of the as-prepared sample. The XPS spectrum for the as-prepared sample indicate that the elements of Ti, O and C were detected. The Ti and O elements result from the titanium plate and H2O. The C element probably comes from the organic surfactant molecule, which are not completely removed from the surface of nanoparticles and the adsorbed carbon dioxide.
The high resolution XPS spectra of the Ti 2p are shown in Fig.6. The Ti 2p region can be decomposed into several contributions corresponding to the different oxidation states of titanium. From Fig.6, the binding energies of Ti 2p3/2 and Ti 2p1/2 are observed to be approximately 458.8 eV and 464.5 eV. The ratio of the area of the two peaks A(Ti 2p1/2)/A(Ti 2p3/2) is equal to 0.5 and the binding energy difference, ΔEb=Eb(Ti2p1/2)- Eb(Ti2p3/2) is 5.7 eV, which is in agreement with the literature[11]. The two peaks are the attribution of Ti4+ (TiO2). Another peak at the binding energy of 460.5 eV is also observed. OCAL[12] believed that the binding energy around 460 eV is associated with Ti2p3/2 in the TiOx/Ti compound. In our case, the appearance of TiOx/Ti is in agreement with the extreme nonequilibrium condition in the PLA process. In the PLA process, the ultrafast oxidation of the highly active titanium clusters can lead to nonequilibrium growth and incomplete oxidation (i.e. oxygen vacancies existed) of the titania, which leads to the formation of TiOx/Ti.

Fig.5 XPS spectrum of as-prepared sample
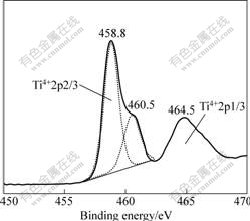
Fig.6 XPS spectra of Ti 2p of sample
3.3 Formation of rutile TiO2 nanoparticles
The high-temperature (about 6 000 K) and high- pressure (about 1 GPa) titanium plasma will be produced on the solid-liquid interface quickly when one pulsed laser shot on the titanium target. Subsequently, the ultrasonic adiabatic expansion of the hot plasma leads to quick cooling of the plume region and hence to the formation of titanium clusters. Finally, with the extinguishment of the plasma, the formed titanium clusters encounter and interact with the solvent and surfactant molecules in the surrounding solution, inducing some chemical reactions. The formation mechanism is similar to that we have studied previously [8]. The process and chemical reaction can be described as follows.
Ti(clusters)+4H2O→Ti(OH)4+2H2 ↑ (1)
Ti(OH)4→TiO2+2H2O (2)
The local high temperature and high pressure in the LAL process can provide such an appropriate conditions for the formation of rutile TiO2.
3.4 Optical properties of rutile TiO2 nanoparticles
Fig.7 shows the ultraviolet-visible (UV-vis) optical absorption spectrum of the obtained colloid solutions. A significant increase in the absorption at wavelengths short than 400 nm is observed.
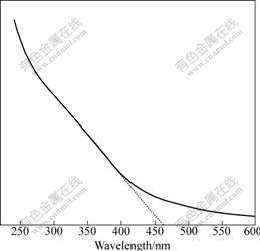
Fig.7 UV-vis absorption spectrum of TiO2 nanoparticle colloidal solution.
Further investigation reveals that the absorbance value in the edge region can well be determined by the optical absorption edge expression of the semiconductor with direct band gap [13]:
αhν=A(hv-Eg)1/2 (3)
where α is the absorbance value; hν is the photon energy of incident light; A is the parameter that relates to the effective masses associated with the valence and conduction bands; Eg is constant. The value of photon energy (hν) extrapolated to α=0 gives an absorption edge energy which corresponds to a band gap Eg. The plot of (αhν)2 vs (hν) is shown in Fig.8. The nature of the plot suggests direct interband transition. The extrapolation gives Eg=3.15 eV. The bulk band gap for rutile is 3.0 eV [14]. This yields a blue shift for the rutile nanoparticles of about 0.15 eV.
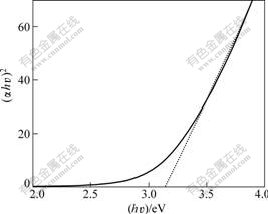
Fig.8 Variation of (αhν)2 with photon energy (hν) for as-prepared TiO2 nanoparticles corresponding to Fig.7
Fig.9 shows the room-temperature PL spectra of the TiO2 nanoparticles, which are taken under an excitation with a line 350 nm. It is seen that these nanoparticles show strong PL band peaks at 416 and 435 nm. The excitation spectra of the emission at 435 and 416 nm at room temperature are presented by curves band in Fig.9. Interestingly, the two excitation spectra exhibit a same peak at 380 nm. These suggest that the two emission peaks at 416 nm and 435 nm originate from same luminescent centers. The peaks at 416 and 435 nm for rutile nanoparticles are not reported in the literature. The authors think that the peak may come from the defects, oxygen vacancies, etc, based on the extreme nonequilibrium growth process of rutile nanoparticles. Further experiments are being investigated for the origin of luminescence.

Fig.9 Room temperature PL spectra (excited at 350 nm) (a), excitation emitting at 435 nm (b) and 416 nm (c) of as-prepared nanoparticles
4 Conclusions
1) The rutile TiO2 nanoparticles are successfully prepared by pulsed laser ablation of pure titanium target in PVP solutions at room temperature.
2) LAL-induced local high temperature and high pressure lead to the formation of rutile TiO2 nanoparticles.
3) This unique fabrication method is an easy way to fabricate rutile nanoparticles at room temperature. The pulse laser ablation in liquid media is a good method to synthesize some nanosized material, such as high-temperature phase material and metastable material, which are difficult to be produced by other conventional methods.
References
[1] ZHOU Y, MA R Z, EBINA Y, TAKADA K, SASAKI T. Multilayer hybrid films of titania semiconductor nanosheet and silver metal fabricated via layer-by-layer self-assembly and subsequent UV irradiation [J]. Chem Mater, 2006, 18: 1235-1239.
[2] TANG H, BERGER H, SCHMID P E, LE?VY F. Photoluminescence in TiO2 anatase single crystals [J]. Solid State Commun, 1993, 87: 847-850.
[3] KUANG D, BRILLET J, CHEN P, TAKATA M, UCHIDA S, MIURA H, SUMIOKA K, ZAKEERUDDIN S M, GRATZEL M. Application of highly ordered TiO2 nanotube arrays in flexible dye-sensitized solar cells [J]. ACS Nano, 2008, 2: 1113-1116.
[4] HOSONO E, FUJIHARA S, IMAI H, HONMA I, MASAKI I, ZHOU H S. One-step synthesis of nano-micro chestnut TiO2 with rutile nanopins on the microanatase octahedron [J]. ACS Nano, 2007, 1: 273-278.
[5] FOX M A, DULAY M T. Heterogeneous photocatalysis [J]. Chem Rev, 1993, 93: 341-357.
[6] DHAGE S R, CHOUBE V D, SAMUEL V, RAVI V. Synthesis of nanocrystalline TiO2 at 100 ℃ [J]. Mater Lett, 2004, 58: 2310-2313.
[7] HU Y, TSAI H L, HUANG C L. Effect of brookite phase on the anatase-rutile transition in titania nanoparticles [J]. J Eur Ceram Soc, 2003, 23: 691-696.
[8] LIU P S, CAI W P, ZENG H B. Fabrication and size-dependent optical properties of FeO nanoparticles induced by laser ablation in a liquid medium [J]. J Phys Chem C, 2008, 112: 3261-3266.
[9] ZENG H B, LIU P S, CAI W P. Aging-induced self-assembly of Zn/ZnO treelike nanostructures from nanoparticles and enhanced visible emission [J]. Cryst Growth Des, 2007, 7: 1092-1097.
[10] USUI H, SASAKI T, KOSHIZAKI N. Effect of alkyl chain length on layered structure of Zn nanocomposites prepared by laser ablation of Zn in aqueous solution of sodium alkyl sulfate [J]. Chem Lett, 2005, 34: 700.
[11] WAGNER C D, RIGGS W M, DAVIS L E, MOULDER J F, MULLENBERG G E. Handbook of X-ray photoelectron spectroscopy [M]. Minnesota:Perkin-Elmer Corporation, 1979: 38.
[12] OCAL C, FERRER. Low temperature diffusion of Pt and Au atoms through thin TiO2 films on a Ti substrate [J]. Surface Science, 1987, 191: 147-156.
[13] MOSS T S. Optical properties of solids [M]. London: Butterworth, 1961: 34.
[14] KANDORI K, KON-NO K, KITAHARA A. Formation of ionic water oil microemulsions and their application in the preparation of CaCO3 particles [J]. J Colloid Interface Sci, 1988, 122: 78-82.
(Edited by LI Yan-hong)
Foundation item: Project(09R23) supported by the Scientific Research Starting Foundation of Nantong University; Projects(50671100, 10604055) supported by the National Natural Science Foundation of China
Corresponding author: LIU Pei-sheng, Tel: +86-513-85012704; E-mail: psliu@ntu.edu.cn