A molecular dynamics study of calcium silicate hydrates-aggregate interfacial interactions and influence of moisture
来源期刊:中南大学学报(英文版)2021年第1期
论文作者:黄家乐 周扬 彭泽川 马涛 黄晓明 缪昌文
文章页码:16 - 28
Key words:calcium silicate hydrate; aggregate; interfacial connections; molecular dynamics simulation; moisture
Abstract: The interface properties between hydrated cement paste (hcp) and aggregates largely determine the various performances of concrete. In this work, molecular dynamics simulations were employed to explore the atomistic interaction mechanisms between the commonly used aggregate phase calcite/silica and calcium silicate hydrates (C-S-H), as well as the effect of moisture. The results suggest that the C-S-H/calcite interface is relatively strong and stable under both dry and moist conditions, which is caused by the high-strength interfacial connections formed between calcium ions from calcite and high-polarity non-bridging oxygen atoms from the C-S-H surface. Silica can be also adsorbed on the dry C-S-H surface by the H-bonds; however, the presence of water molecules on the interface may substantially decrease the affinities. Furthermore, the dynamics interface separation tests of C-S-H/aggregates were also implemented by molecular dynamics. The shape of the calculated stress-separation distance curves obeys the quasi-static cohesive law obtained experimentally. The moisture conditions and strain rates were found to affect the separation process of C-S-H/silica. A wetter interface and smaller loading rate may lead to a lower adhesion strength. The mechanisms interpreted here may shed new lights on the understandings of hcp/aggregate interactions at a nano-length scale and creation of high performance cementitious materials.
Cite this article as: ZHOU Yang, PENG Ze-chuan, HUANG Jia-le, MA Tao, HUANG Xiao-ming, MIAO Chang-wen. A molecular dynamics study of calcium silicate hydrates-aggregate interfacial interactions and influence of moisture [J]. Journal of Central South University, 2021, 28(1): 16-28. DOI: https://doi.org/10.1007/s11771-021-4582-4.

J. Cent. South Univ. (2021) 28: 16-28
DOI: https://doi.org/10.1007/s11771-021-4582-4
ZHOU Yang(周扬)1, 2, PENG Ze-chuan(彭泽川)1, 2, HUANG Jia-le(黄家乐)1, 2,
MA Tao(马涛)3, HUANG Xiao-ming(黄晓明)3, MIAO Chang-wen(缪昌文)1, 2
1. School of Materials Science and Engineering, Southeast University, Nanjing 211189, China;
2. State Key Laboratory of High Performance Civil Engineering Materials, Jiangsu Research Institute of Building Science Co., Nanjing 211103, China;
3. School of Transportation, Southeast University, Nanjing 211189, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract: The interface properties between hydrated cement paste (hcp) and aggregates largely determine the various performances of concrete. In this work, molecular dynamics simulations were employed to explore the atomistic interaction mechanisms between the commonly used aggregate phase calcite/silica and calcium silicate hydrates (C-S-H), as well as the effect of moisture. The results suggest that the C-S-H/calcite interface is relatively strong and stable under both dry and moist conditions, which is caused by the high-strength interfacial connections formed between calcium ions from calcite and high-polarity non-bridging oxygen atoms from the C-S-H surface. Silica can be also adsorbed on the dry C-S-H surface by the H-bonds; however, the presence of water molecules on the interface may substantially decrease the affinities. Furthermore, the dynamics interface separation tests of C-S-H/aggregates were also implemented by molecular dynamics. The shape of the calculated stress-separation distance curves obeys the quasi-static cohesive law obtained experimentally. The moisture conditions and strain rates were found to affect the separation process of C-S-H/silica. A wetter interface and smaller loading rate may lead to a lower adhesion strength. The mechanisms interpreted here may shed new lights on the understandings of hcp/aggregate interactions at a nano-length scale and creation of high performance cementitious materials.
Key words: calcium silicate hydrate; aggregate; interfacial connections; molecular dynamics simulation; moisture
Cite this article as: ZHOU Yang, PENG Ze-chuan, HUANG Jia-le, MA Tao, HUANG Xiao-ming, MIAO Chang-wen. A molecular dynamics study of calcium silicate hydrates-aggregate interfacial interactions and influence of moisture [J]. Journal of Central South University, 2021, 28(1): 16-28. DOI: https://doi.org/10.1007/s11771-021-4582-4.
1 Introduction
The cement and concrete industry, manufacturing the most widely applied civil engineering materials in the world, accounts for 6%-8% CO2 footprint annually. Therefore, creating high performance cement-based materials, which can effectively decrease the material consumption at the same loading capacity, is of great significance to fulfill a low-carbon sustainable development [1]. Concrete is a multiphase composite, mainly composed of cement hydration products, aggregates, and a third phase in between, called as the interface transition zone (ITZ). The ITZ situates at the interface between cement paste and aggregates, with considerably higher porosity than the matrix, which largely determines the mechanical properties and durability of the whole composite [2]. FARRAN [3] proved that the ITZ is a key region for the formation and propagation of cracks under external loadings.
Furthermore, the microstructure and performance of ITZ are mainly dependent on the interfacial interactions between hydration products and aggregate, which are contributed by the surface chemical reaction, van der Waals force, and interlocking effect. The bonding strength was normally utilized to evaluate and quantify the interface stability between hydrated cement paste and aggregate. This value can be measured by direct tensile tests [4], direct shear tests [5], as well as compression and splitting tests [6], according to previous studies. However, most of the studies mentioned above focused on the macroscopic performances and the corresponding conclusions about the interface were almost empirical. On the other hand, although the composition and porosity of ITZ can be illustrated from a microscopic level [7-11], the cementitious matrix is composed of various hydration products, which may have distinct interactions with the aggregates. Calcium silicate hydrate (C-S-H), the basic and largest volume proportion hydration product, should be considered in priority. So far, publications dealing with the detailed interaction mechanisms between aggregate and C-S-H are still missing, due to the lack of high-resolution techniques to access an adequately small length scale.
Molecular dynamics (MD) simulation, a numerical computation method based on force fields and Newtonian kinetics, has been an effective tool to investigate the composition, structure, energy and mechanical properties of cement-based materials, and has contributed to elucidating the interfacial interactions between hydration products and pore solutions at the molecular scale [12-15]. It is believed that MD can also provide one new point of view to interpret the interaction mechanisms at the C-S-H/aggregates interfaces, which are experimentally inaccessible due to the resolution limitations of normal laboratory techniques. In previous studies, MD has illustrated the basic molecular composition, structure, and mechanical properties of C-S-H, which achieved a high degree agreement with the measurements of high pressure X-ray diffraction (HP-XRD) and 29Si nuclear magnetic resonance (NMR) [16-20]. By MD simulations, the transport behaviors of chloride ions in the nano-pores of C-S-H were investigated, while the adsorption mechanisms in the C-S-H/ aqueous solution interfaces also unraveled and the adsorption amount was determined by the surface calcium to silicon ratio of C-S-H [21, 22]. Besides, MD has also been used to give molecular insights into the C-S-H/polymer interface [23-25] and asphalt/aggregate interface [26-28]. The detailed interactions between polymer chains with different functional groups and C-S-H were illustrated [29]. According to the simulation results, reliable criteria were proposed to guarantee a stable adsorption of polymers on the surface of hydrated cement particles, which was consistent with an experimental study [30]. Furthermore, in asphalt/ aggregate interface, which largely determines the mechanical performance of asphalt concrete, the adhesion property of two types of aggregate phase (calcite and silica) on the matrix was investigated, and the interface separation loading tests were also simulated. Silica and calcite are two representative minerals of aggregate in concrete. For example, granite, basalt, sandstone and sand consist principally of silica. While calcite is common in limestone, marble, and dolomite. It needs to be noted that with respect to the chemical composition, calcite is a typical alkaline aggregate and silica is naturally acidic. It is expected that hydration products may exhibit distinct performances on the surface of calcite or silica.
Therefore, in this work, molecular dynamics simulations were employed to distinguish the interfacial interactions between cementitious matrix and acid/alkaline aggregates. The model of C-S-H was simplified as a matrix since it is the main hydration product, while the crystal structure of calcite/silica was established to denote aggregates. The influences of moisture degree on the adhesion properties of C-S-H/calcite and C-S-H/silica interfaces were also investigated. Furthermore, the dynamic stability of interfaces was also evaluated by simulating a phase-separation test at a fixed tensile strain rate.
2 Molecular dynamics simulation method
2.1 Molecule models of C-S-H
C-S-H is a complex porous material with multi-scale feature and at nano-scale it is constituted with alternating calcium-silicate sheets. In the interlayer of C-S-H, it is filled with water molecules and calcium hydroxyls and silanols [31]. RICHARDSON [32] found that the crystal structure of C-S-H is similar to Tobermorite and Jennite by the techniques of TEM and XRD. This investigation used a crystal structure of 11  Tobermorite as the origin model. Firstly, all of the interlayer water molecules were removed. Secondly, partial structure of bridging SiO2 was deleted and calcium ions were added into the crystal structure, so as to increase the model’s calcium-silicon ratio from 1 to 1.3 and to simultaneously guarantee the Q distribution of silicon chain structure approximately consistent with the results of 29Si MAS NMR [33]. The unit cell of C-S-H was built with lattice parameters of a=11.16
Tobermorite as the origin model. Firstly, all of the interlayer water molecules were removed. Secondly, partial structure of bridging SiO2 was deleted and calcium ions were added into the crystal structure, so as to increase the model’s calcium-silicon ratio from 1 to 1.3 and to simultaneously guarantee the Q distribution of silicon chain structure approximately consistent with the results of 29Si MAS NMR [33]. The unit cell of C-S-H was built with lattice parameters of a=11.16  , b=7.39
, b=7.39  , c=22.77
, c=22.77  , α=β=γ=90°, as shown in Figure 1(a). Then a C-S-H supercell which should be almost close to the size of the gel pores, typically 0.5-1.0 nm, was established with dimensions of 44.64
, α=β=γ=90°, as shown in Figure 1(a). Then a C-S-H supercell which should be almost close to the size of the gel pores, typically 0.5-1.0 nm, was established with dimensions of 44.64  ×44.34
×44.34  × 45.54
× 45.54  by repeating the lattice. This size is the least common multiple of the size of the smallest unit cell of C-S-H. Finally, Grand Canonical
by repeating the lattice. This size is the least common multiple of the size of the smallest unit cell of C-S-H. Finally, Grand Canonical
Monte Carlo water absorption simulation was performed to fix the chemical potential of water molecular to the standard state (298 K, 1 g/cm3) till the C-S-H molecular model reaches water saturation.
2.2 Molecule models of aggregate
In this study, two kinds of crystal structures, silica and calcite, are used to represent the aggregate, due to their representative chemical compositions. These minerals are widely used as aggregate in concrete. A unit cell of silica was imported from structures database in Materials Studio 8.0 directly, which has lattice parameters of a=b=4.978  , c=6.948
, c=6.948  , α=β=γ=90°, as shown in Figure 1(b). While for the calcite, at first, a unit cell of calcite with lattice parameters of a=b=4.990
, α=β=γ=90°, as shown in Figure 1(b). While for the calcite, at first, a unit cell of calcite with lattice parameters of a=b=4.990  , c=17.061
, c=17.061  , α=β=90°, γ=120° was imported. Then, to transform it to an orthogonal geometry, the smallest periodic unit was selected from the calcium carbonate supercell. Finally, a unit cell of calcite with lattice parameters of a=8.643
, α=β=90°, γ=120° was imported. Then, to transform it to an orthogonal geometry, the smallest periodic unit was selected from the calcium carbonate supercell. Finally, a unit cell of calcite with lattice parameters of a=8.643  , b=4.990
, b=4.990 , c=17.061
, c=17.061  , α=β=γ=90° was obtained, as shown in Figure 2.
, α=β=γ=90° was obtained, as shown in Figure 2.

Figure 1 Schematic illustrasion of models of C-S-H, silica and calcite (red: oxygen atoms; orange: silicon atoms; green: calcium atoms; white: hydrogen atoms; gray: carbon atoms):

Figure 2 Pick-up of smallest periodic unit from a calcium carbonate supercell (z direction perpendicular to paper) (red: oxygen atoms; green: calcium atoms; gray: carbon atoms)
2.3 Creation of different C-S-H/aggregate interfaces
To meet the size of C-S-H, one supercell of aggregate was set up. In the beginning, the unit silica or calcite was cleaved along [0, 0, 1] direction with a thickness of 48  . Then, the unit cell was repeated along x- and y-direction until the size of C-S-H. Subsequently, a 42
. Then, the unit cell was repeated along x- and y-direction until the size of C-S-H. Subsequently, a 42  vacuum slab was located in the top to avoid the untended interactions induced by a periodic boundary along the z-direction. As a whole, the C-S-H/silica interface model has dimensions of a=45
vacuum slab was located in the top to avoid the untended interactions induced by a periodic boundary along the z-direction. As a whole, the C-S-H/silica interface model has dimensions of a=45  , b=45
, b=45  , c=135
, c=135  and the C-S-H/calcite interface model has dimensions of a=44.64
and the C-S-H/calcite interface model has dimensions of a=44.64  , b=44.34
, b=44.34  , c=135
, c=135  . The two interfaces are shown in Figure 3.
. The two interfaces are shown in Figure 3.
2.4 Force fields and simulation details
Two force fields, clay force field (CLAYFF) and consistent valence force field (CVFF), were employed in this work. The CLAYFF was chosen for the C-S-H while the CVFF was selected for the aggregates. The CLAYFF force field has been successfully used to the structures of oxide and hydroxide materials, the interactions of aqueous species with oxide and hydroxide materials’ surfaces, and the behavior of water and ionic species in the interlayers of layered structures [34]. The CLAYFF is based on nonbonded interactions of the metal-oxygen in hydrated phases [14] and has been widely employed to describe the interactions among calcium, silicate, oxygen and hydrogen atoms of C-S-H [22, 35, 36]. All atoms are regarded as point charges and the empirical parameters are calculated on the basis of the density function theory (DFT) [37]. In addition, single point charge (SPC) water model was used to describe the dynamics of H2O and OH- [38]. For the aggregates, the CVFF force field was chosen, which is a consistent valence force field and was originally created to reproduce peptide and protein properties [39, 40]. It can also be used to deal with many other systems. The potential states related to bond lengths, bond angles, torsion angles and out-of-plane interactions can be precisely predicted by CVFF. The interaction parameters between organic and inorganic atoms are determined according to the mean rule. Distance parameters are calculated by arithmetic mean rule, while energy parameters are calculated by geometric mean rule [29, 41].
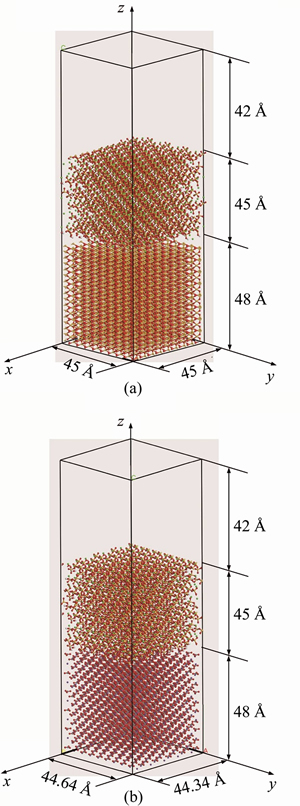
Figure 3 Models of C-S-H/silica interface and C-S-H/calcite interface (red: oxygen atoms; orange: silicon atoms; green: calcium atoms; white: hydrogen atoms; gray: carbon atoms)
All models were established by Material Studio 8.0 in the platform of the High-performance Computing Centre in Nanjing University, China. Large-scale atomic/molecular massively parallel simulator (LAMMPS) was employed to carry out the simulations. For each of the simulations, the combined CLAYFF/CVFF force field was set first, then the model was relaxed until equilibrium or energy minimization was attained. A periodic boundary was applied to x, y and z directions. The nose-hoover canonical ensemble (NVT) was employed at 298 K, with a time step of 1 fs. Firstly, a 500 ps run was performed after equilibrium and data was collected every 1 ps to investigate the interfacial interactions between C-S-H and aggregates after a 100 ps equilibration time. Secondly, a pull-out test was implemented to simulate the dynamic separation process of interface phases. In this simulation, the vacuum slab at the top of the interface model (Figure 3) was removed. The C-S-H model was fixed while the aggregate model at the bottom was moved downward, away from the C-S-H at a constant velocity. The interface was separated since the movement of C-S-H was limited. In the pull-out process, traction forces and coordinates of all atoms were recorded every 1 ps. Tensile stresses were calculated by normalizing the traction force by the interface area, and the stress-strain curves during the whole process were plotted.
3 Results and discussion
In this section, firstly, the interaction energy of C-S-H/calcite and C-S-H/silica was compared to show the adhesion properties of alkaline and acid aggregates on the C-S-H matrix. Besides, the moisture influence on the interaction energy was discussed. Secondly, the detailed interaction mechanisms were proposed to explain the different adhesion properties. Finally, a pull-out test was simulated to evaluate the dynamics interface stability of C-S-H/calcite and C-S-H/silica, and the effects of moisture and loading rates were discussed.
3.1 Interaction energy
The interaction energy between C-S-H and aggregate is a significant indicator which can measure the repulsion and attraction between two phases. In molecular scale, the energy can be regarded as an intermolecular combination parameter composed of covalent bond, van der Waals and electrostatic energy, H bonds and so on, indicating the energy required to separate aggregate from the surface of C-S-H. Here, the interaction energy between aggregate and C-S-H can be calculated according to Eq. (1), which has been calculated by work of adhesion between aggregate and asphalt [27, 42, 43]; the interaction energy between aggregate and water can be calculated with Eq. (2) and that between C-S-H and water can be obtained with Eq. (3). If the calculated interaction energy between aggregate and C-S-H is negative, it means that the two phases attract each other; otherwise, it is a repulsion interface. A larger absolute value indicates a stronger interaction for both attraction and repulsion. Besides, it is believed that moisture content may affect the interface feature, and thus a parameter of energy ratio (ER, nER) is proposed to evaluate the influence of saturation degree on the interface affinity shown in Eq. (4) [42]. A higher value of nER indicates a stronger and more stable interface.
 (1)
(1)
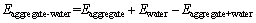 (2)
(2)
 (3)
(3)
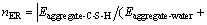
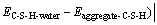 (4)
(4)
where 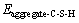 is interaction energy between aggregate and C-S-H; Eaggregate is potential energy of aggregate exited lonely; EC-S-H is potential energy of C-S-H exited lonely; Eaggregate+C-S-H is systematic potential energy of aggregate-C-S-H; Eaggregate-water is interaction energy between aggregate and water; Ewater is potential energy of water; Eaggregate+water is systematic potential energy of aggregate-water; EC-S-H+water is systematic potential energy of C-S-H-water; EC-S-H+water is interaction energy between C-S-H and water; and nER is the ratio of the function of adhesion between aggregate and C-S-H to the work of debonding when water appears in the interface area.
is interaction energy between aggregate and C-S-H; Eaggregate is potential energy of aggregate exited lonely; EC-S-H is potential energy of C-S-H exited lonely; Eaggregate+C-S-H is systematic potential energy of aggregate-C-S-H; Eaggregate-water is interaction energy between aggregate and water; Ewater is potential energy of water; Eaggregate+water is systematic potential energy of aggregate-water; EC-S-H+water is systematic potential energy of C-S-H-water; EC-S-H+water is interaction energy between C-S-H and water; and nER is the ratio of the function of adhesion between aggregate and C-S-H to the work of debonding when water appears in the interface area.
Figure 4 shows the calculated interaction energy between aggregate and C-S-H in different moisture conditions. The negative values suggest that both C-S-H/silica and C-S-H/ calcite pairs are attractive. Also, it is found that the affinity energy of C-S-H/calcite is substantially greater than that of C-S-H/silica, which indicates that calcite has a higher affinity with the C-S-H matrix. Mechanisms will be explained later. On the other hand, Figure 5 shows the calculated ER values of adhesion between C-S-H/silica and C-S-H/calcite in three moist conditions, i.e. dry, half-moist and moist. The completely dry state is to observe the interface connection characteristics of C-S-H and aggregates on the nanometer scale. The half-moist condition is to simulate the actual situation of the interface characteristics between C-S-H and aggregate inside the concrete. However, the degree of moist is a statistically significant variable. The number of water molecules at the interface in the half-moist condition is half of the number of water molecules at the interface in the moist condition to facilitate model analysis. The moist condition is to reflect the wetting contact between the C-S-H and the aggregate interface area. Water can fill the pits of the solid rough surface to form a wetted surface, making it be in a fully moist condition based on the wenzel theory. The absolute adhesion energy of C-S-H/silica interface decreases and ER deceases dramatically as the moisture content increases. It means the presence of water molecules damages the C-S-H/silica interface which is consistent with some experiments at the macroscopic scale [44, 45]. However, it should be noted that the influence of moisture on C-S-H/calcite interface adhesive strength and ER is completely different. In the latter case, with the absolute adhesion energy and the ER increasing, the introduction of water molecules seems to improve the interface affinities between C-S-H and calcite. The same finding is also observed from experimental studies. VARGA et al [10] investigated the effect of interface moisture on the tensile bond strength and grout microstructure, and the results show increased tensile bond strength when the concrete surface is pre-moistened. In conclusion, calcite is more prone to adsorb on the C-S-H surface at both dry and moisture conditions, while a more humid interface seems to weaken the C-S-H/silica interactions.
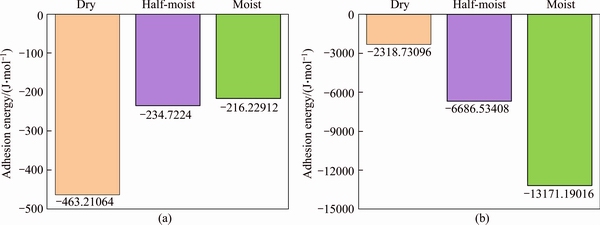
Figure 4 Interaction energy of C-S-H/silica (a) and C-S-H/calcite interface (b)
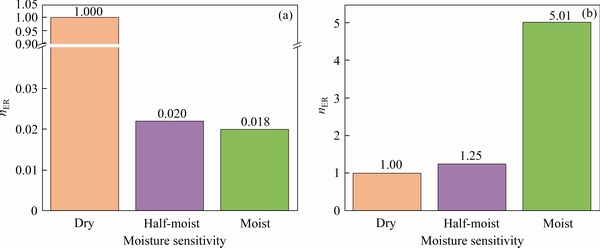
Figure 5 Moisture sensitivity of C-S-H/silica (a) and C-S-H/calcite (b) interaction with nER values calculated from MD simulation
3.2 Interfacial interaction mechanisms
The difference in the interaction energy should result from the interaction mechanisms between atoms from aggregates and the C-S-H surface. The radial distribution function (RDF), which deals with the spatial atomic correlations, can provide abundant structural information for the C-S-H/ aggregate interface. The RDF (gAB(r)) is defined as the probability of finding an atom B located at a distance r from an atom A, calculated using Eq. (5).
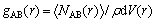 (5)
(5)
where gAB(r) represents the probability of the B atom appearing at r around the A atom;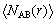 is the average number of ensembles of B atoms from r to r+dr around A atoms; ρ is the density of B atoms; and dV(r) is the volume of the spherical sphere shell from r to r+dr around A atom.
is the average number of ensembles of B atoms from r to r+dr around A atoms; ρ is the density of B atoms; and dV(r) is the volume of the spherical sphere shell from r to r+dr around A atom.
The interface connections captured by RDF on C-S-H/silica and C-S-H/calcite interfaces are shown in Figure 6. On the one hand, for C-S-H/silica interface, there is an obvious peak located at around 2.75  , which indicates that HC-S-H from Si—OH groups on C-S-H surface and Osilica from silica surface form atom pairs at this distance. The HC-S-H-Osilica atom pairs can be viewed from the interface snapshot in Figure 7, where the high- polarity non-bridging oxygen atoms of silica can attract the hydroxyl groups of C-S-H, forming a H-bond connection. On the other hand, at a shorter distance (around 1.55
, which indicates that HC-S-H from Si—OH groups on C-S-H surface and Osilica from silica surface form atom pairs at this distance. The HC-S-H-Osilica atom pairs can be viewed from the interface snapshot in Figure 7, where the high- polarity non-bridging oxygen atoms of silica can attract the hydroxyl groups of C-S-H, forming a H-bond connection. On the other hand, at a shorter distance (around 1.55  ), CaC-S-H from C-S-H surface coordinates with Ocalcite from calcite surface on C-S-H/calcite interface. The snapshot in Figure 8 illustrates the interaction details. The high-polarity non-bridging oxygen atoms of C-S-H strongly attract the positively charge calcium ions of calcite, forming an ionic bond. The bond distance of CaC-S-H-Ocalcite is significantly lower than that of HC-S-H-Osilica pairs, implying a higher bond energy. It contributes to the higher interaction energy on C-S-H/calcite interface, as mentioned above.
), CaC-S-H from C-S-H surface coordinates with Ocalcite from calcite surface on C-S-H/calcite interface. The snapshot in Figure 8 illustrates the interaction details. The high-polarity non-bridging oxygen atoms of C-S-H strongly attract the positively charge calcium ions of calcite, forming an ionic bond. The bond distance of CaC-S-H-Ocalcite is significantly lower than that of HC-S-H-Osilica pairs, implying a higher bond energy. It contributes to the higher interaction energy on C-S-H/calcite interface, as mentioned above.

Figure 6 RDF on C-S-H/silica and C-S-H/calcite interfaces
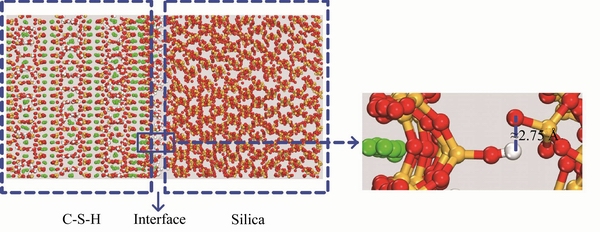
Figure 7 Snapshot of interface between C-S-H and silica (red: oxygen atoms, orange: silicon atoms; green: calcium atoms; white: hydrogen atoms)
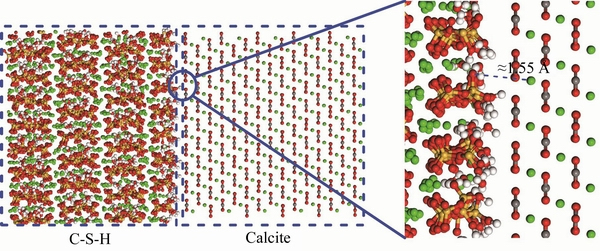
Figure 8 Snapshot of interface between C-S-H and calcite (red: oxygen atoms, orange: silicon atoms; green: calcium atoms; white: hydrogen atoms; gray: carbon atoms)
3.3 Interface adhesive stress during interface separation process
The dynamic interface separation behavior was also investigated in addition to the static analysis of interaction energy RDF. During the interface separation process, the total traction force on the aggregate is recorded, while the adhesive stress between C-S-H and aggregate can be calculated using Eq. (6).
 (6)
(6)
where σ is interface adhesive stress; F is total traction force; A is interface contact area.
The typical MD prediction of interface tensile stress at C-S-H/silica and C-S-H/calcite interface during the interface separation process is shown in Figures 9 and 10. The set loading rate was 1 m/s and the stress data were extracted every 20 ps during the separation process. For C-S-H/silica interface, it can be seen that surface atoms of C-S-H were disorderly distributed due to the attraction effect of the silica as it was displacing apart from C-S-H. The stress-separation curve exhibits one nearly linear relationship until stress reached the first peak. The first peak corresponds to about 800 MPa at 0.7  separation. Next, the stress reduced quickly until the presence of another minor peak. This means that the interactions between two phases were diminishing. Subsequently, some fluctuations occur due to thermal contributions [27]. After about 7.0
separation. Next, the stress reduced quickly until the presence of another minor peak. This means that the interactions between two phases were diminishing. Subsequently, some fluctuations occur due to thermal contributions [27]. After about 7.0  separation, the interface stress reduced to zero and maintained stable, indicating a complete interface separation. The C-S-H/calcite interface has a similar stress-separation relationship with the C-S-H/silica interface. The stress- separation exhibited nearly linear relationship until 0.2
separation, the interface stress reduced to zero and maintained stable, indicating a complete interface separation. The C-S-H/calcite interface has a similar stress-separation relationship with the C-S-H/silica interface. The stress- separation exhibited nearly linear relationship until 0.2  separation with a maximal stress of 265 MPa. Since the separation reached 7.0
separation with a maximal stress of 265 MPa. Since the separation reached 7.0  , the stress reduced to zero and maintained stable. It should be noted that the shape of interface stress-separation curve calculated in MD simulations obeys the quasi-static cohesive law obtained from the macroscopic experimental results [46].
, the stress reduced to zero and maintained stable. It should be noted that the shape of interface stress-separation curve calculated in MD simulations obeys the quasi-static cohesive law obtained from the macroscopic experimental results [46].
However, the adhesion strength (the maximal stress of the stress-separation curve) obtained from MD simulations is two orders of magnitude greater than experimental results. JEBLI et al [4] studied the cement-aggregate interface by placing 2 mm cement paste between two natural limestone parallelepipedic aggregates with dimensions of 10 mm×10 mm×15 mm, and obtained an interface adhesion strength of 1.6 MPa by direct tensile measurements. VARGA et al [10] built a cement- aggregate interface by exposing the coarse aggregate consisting of dolomitic limestone to cement paste, while the pull-off bond strength measured for various batches was about 2-3 MPa. This big difference is largely due to the different length scale involved, independent of the simulations or experiments. The modulus or strength of materials always decreases as a result of an increase in the length scale [26]. Furthermore, it can also be attributed to the temporal and spatial limitations of MD simulations. The atomistic scale calculations fail to illustrate the influence of defects at a larger length, i.e. the C-S-H pores and aggregate texture differences [47]. Besides the MD simulations results are also dependent on the other setting parameters, e.g. model size, force field type and simulation time. The influence of moisture contents and strain rates on the interface adhesion stress between C-S-H and aggregate will be discussed as follows.

Figure 9 Schematic illustration of interface separation process of C-S-H/silica subjected to a pull-out test

Figure 10 Schematic illustration of interface separation process of C-S-H/calcite subjected to a pull-out test
Based on the influence of humidity on cement-based materials [48, 49], three interface models with different dosage of water molecules at the C-S-H/silica interface were set up for MD simulations, named dry, half-moist and moist. The effects of moisture contents on the first peak in the stress-separation curve are shown in Figure 11. Obviously, it is observed that the introduction of water molecules decreases the adhesive strength at the interface. In the meanwhile, the position of the first peak shifts to a lower separation distance with an increase of moisture contents. It implies that at moisture conditions the C-S-H/silica interface is weaker than one at dry condition, which is consistent with the above findings with respect to the interaction energy. Also, this trend is similar with that observed in some macroscopic experiments. BEUSHAUSEN et al [45] suggested that pre-wetting the substrate surface presented negative influence on the interface bond strength. SANCHEZ et al [50] also pointed out the average wet-bond strength value was approximately 0.54-0.68 times that of the dry-bond strength value.
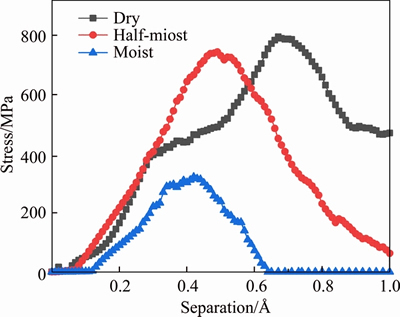
Figure 11 Effects of moisture contents on interface stress-separation relationships at C-S-H/silica interface
The loading rates utilized in MD simulations are always greater than those applied in macroscopic measurements due to the high computation cost. As described earlier, this may cause deviations between calculations and experiments. Therefore, velocities of 1, 2, 5 and 10 m/s are equivalent to strain rates of 6.9×107, 1.38×108, 3.45×108 and 6.9×108 s-1, respectively, with respect to the original length of C-S-H/ aggregate model configuration. These velocities were chosen to evaluate this effect of loading regimes, as shown in Figure 12. As for the C-S-H/silica interface, the strain rates hardly affect the overall pattern of the stress-separation curve. However, it is observed that the higher the loading rate was employed, the greater the value of adhesion strength was gained (800 MPa for velocity of 1 m/s and 1040 MPa velocity of 10 m/s). The macroscopic tensile loading experiments also supported this trend [51-53]. It suggested that smaller loading rates usually caused a lower strength since the propagation of cracks acquired sufficient time. Furthermore, the peak position of the first peak shifted to the left as a result of an increasing strain rates.
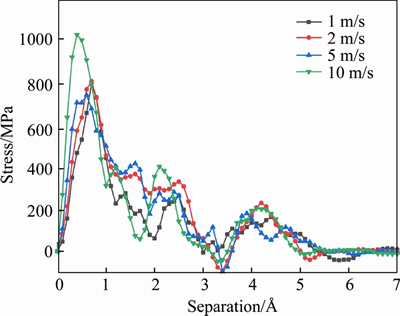
Figure 12 Effects of loading rates on interface stress-separation relationships at C-S-H/silica interface
4 Conclusions
1) In this work, molecular dynamics simulations are employed to compare the interface properties of C-S-H and aggregates (calcite or silica), and evaluate the influence of moisture. The results indicate that both interfaces are attracted and the C-S-H/calcite interface has a higher affinity. Besides, the moisture condition hardly affects the stability of C-S-H/calcite interface, while the attraction energy between gels and silica decreases due to the incorporation of water molecules. The differences in the adhesion performance are caused by the respective atomistic connections on the interfaces.
2) Furthermore, the dynamic separation behaviors of C-S-H/silica and C-S-H/calcite interfaces are also investigated by MD simulations. The interface adhesive stress was recorded as the aggregate was displacing away from the C-S-H matrix at a fixed rate. The shapes of the calculated stress-separation curves on both interfaces are similar, which obey the quasi-static cohesive law obtained experimentally. The moisture conditions and strain rates are found to affect the separation process of C-S-H/silica. A wetter interface and smaller loading rate may lead to a lower adhesion strength.
3) This investigation shows that MD can help understand hcp/aggregate interfacial interactions and the dynamic mechanical response of composites subjected to pull-out tests at a nano-length scale, as well as the influence of moisture condition. It may be beneficial to the selection of aggregates, strengthening of interfacial transition zone, and creation of high performance cementitious materials, which leads to a low-carbon and sustainable cement and concrete industry.
Contributors
The overarching research goals were developed by ZHOU Yang, PENG Ze-chuan, HUANG Jia-le, MA Tao, HUANG Xiao-ming and MIAO Chang-wen. ZHOU Yang, MA Tao, HUANG Xiao-ming and MIAO Chang-wen contributed to the conception of the study. PENG Ze-chuan and HUANG Jia-le designed and performed the simulations. ZHOU Yang and HUANG Jia-le performed the data analysis and wrote the manuscript. ZHOU Yang and HUANG Jia-le replied to reviewers’ comments and revised the final version.
Conflict of interest
ZHOU Yang, PENG Ze-chuan, HUANG Jia-le, MA Tao, HUANG Xiao-ming and MIAO Chang-wen declare that they have no conflict of interest.
References
[1] HAJEK P. Concrete structures for sustainability in a changing world [J]. Procedia Engineering, 2017, 171: 207-214. DOI: 10.1016/j.proeng.2017.01.328.
[2] ZHU Zhi-gang, CHEN Hui-su. Overestimation of ITZ thickness around regular polygon and ellipse aggregate [J]. Computers and Structures, 2017, 182: 205-218. DOI: 10.1016/j.compstruc.2016.11.015.
[3] FARRAN J. Introduction: The transition zone-discovery and development [R]. London: E&FN SPON, 1996.
[4] JEBLI M, JAMIN F, MALACHANNE E, GARCIA-DIAZ E, YOUSSOUFI M S. Experimental characterization of mechanical properties of the cement-aggregate interface in concrete [J]. Construction and Building Materials, 2018, 161: 16-25. DOI: 10.1016/j.conbuildmat.2017.11.100.
[5] NESRINE S, MOUAD J, ETIENNE M, FREDERIC J, FREDERIC D, ANNE-SOPHIE C, ERIC G D, MOULAY SAID E Y. Identification of a cohesive zone model for cement paste-aggregate interface in a shear test [J]. European Journal of Environmental and Civil Engineering, 2019, online. DOI: 10.1080/19648189.2019.1623082.
[6] PENG Hui, CUI Chao, CAI C S, LIU Yang, LIU Zhen. Microstructure and microhardness property of the interface between a metakaolin/GGBFS-based geopolymer paste and granite aggregate [J]. Construction and Building Materials, 2019, 221: 263-273. DOI: 10.1016/j.conbuildmat.2019. 06.090.
[7] HE Shan, LI Zhong, YANG En-hua. Quantitative characterization of anisotropic properties of the interfacial transition zone (ITZ) between microfiber and cement paste [J]. Cement and Concrete Research, 2019, 122(8): 136-146. DOI: 10.1016/j.cemconres.2019.05.007.
[8] SHEN Qi-zhen, PAN Gang-hua, ZHAN Hua-gang. Effect of interfacial transition zone on the carbonation of cement-based materials [J]. Journal of Materials in Civil Engineering, 2017, 29(7): 1-9. DOI: 10.1061/(ASCE)MT. 1943-5533.0001860.
[9] LYU Kai, SHE Wei. Determination of aggregate surface morphology at the interfacial transition zone (ITZ) [J]. Journal of Visualized Experiments: JoVE, 2019(154): 1-9. DOI: 10.3791/60245.
[10] de la VARGA I, MUNOZ J F, BENTZ D P, SPRAGG R P, STUTZMAN P E, GRAYBEAL B A. Grout-concrete interface bond performance: Effect of interface moisture on the tensile bond strength and grout microstructure [J]. Construction and Building Materials, 2018, 170: 747-756. DOI: 10.1016/j.conbuildmat.2018.03.076.
[11] ZHOU Yang, SHE Wei, HOU Dong-shuai, YIN Bing, CHANG Hong-lei, JIANG Jin-yang, LI Jia-qi. Modification of incorporation and in-situ polymerization of aniline on the nano-structure and meso-structure of calcium silicate hydrates [J]. Construction and Building Materials, 2018, 182: 459-468. DOI: 10.1016/j.conbuildmat.2018.06.141.
[12] MURRAY S J, SUBRAMANI V J, SELVAM R P, HALL K D. Molecular dynamics to understand the mechanical behavior of cement paste [J]. Transportation Research Record, 2010, 2142(1): 75-82. DOI: 10.3141/2142-11.
[13] HAJILAR S, SHAFEI B. Mechanical failure mechanisms of hydrated products of tricalcium aluminate: A reactive molecular dynamics study [J]. Materials and Design, 2016, 90: 165-176. DOI: 10.1016/j.matdes.2015.10.089.
[14] CYGAN R T, LIANG Jian-jie, KALINICHEV A G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field [J]. Journal of Physical Chemistry B, 2004, 108(4): 1255-1266. DOI: 10.1021/jp0363287.
[15] LI Zhi-hui, LIU Wen-tao, LI Zhong-yuan, DUAN Xiang-yuan, GAO Xu-jing, LI Yun-cai, YANG Ming-cheng, HE Su-qin, ZHU Cheng-shen. Swelling properties and molecular simulation of PNIPA porous hydrogels [J]. Journal of Central South University, 2013, 20(5): 1161-1172. DOI: 10.1007/s11771-013-1599-3.
[16] PELLENQ R J M, KUSHIMA A, SHAHSAVARI R, van VLIET K J, BUEHLER M J, YIP S, ULM F J. A realistic molecular model of cement hydrates [J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(38): 16102-16107. DOI: 10.1073/pnas. 0902180106.
[17] ALLEN A J, THOMAS J J, JENNINGS H M. Composition and density of nanoscale calcium–silicate–hydrate in cement [J]. Nature Materials, 2007, 6(4): 311-316. DOI: 10.1038/ nmat1871.
[18] ZHOU Yang, OROZCO C A, DUQUER E, MANZANO H, GENG Guo-qing, FENG Pan, MONTEIRO P J M, MIAO Chang-wen. Modification of poly (ethylene glycol) on the microstructure and mechanical properties of calcium silicate hydrates [J]. Cement and Concrete Research, 2019, 115: 20-30. DOI:10.1016/j.cemconres.2018.10.001.
[19] GENG Guo-qing, LI Jia-qi, ZHOU Yang, LIU Lin, YAN Jin-yuan, KUNZ M, MONTEIRO P J M. A high-pressure X-ray diffraction study of the crystalline phases in calcium aluminate cement paste [J]. Cement and Concrete Research, 2018, 108: 38-45. DOI: 10.1016/j.cemconres.2018.03.004.
[20] LIU Gui-hua, ZHANG Wen, QI Tian-gui, PENG Zhi-hong, ZHOU Qiu-sheng, LI Xiao-bin. Influence of silicate anions structure on desilication in silicate-bearing sodium aluminate solution [J]. Journal of Central South University, 2016, 23(7): 1569-1575. DOI: 10.1007/s11771-016-3210-1.
[21] ZHOU Yang, HOU Dong-shuai, JIANG Jin-yang, LIU Lin, SHE Wei, YU Jiao. Experimental and molecular dynamics studies on the transport and adsorption of chloride ions in the nano-pores of calcium silicate phase: The influence of calcium to silicate ratios [J]. Microporous and Mesoporous Materials, 2018, 255: 23-35. DOI: 10.1016/j.micromeso. 2017.07.024.
[22] ZHOU Yang, HOU Dong-shuai, JIANG Jin-yang, WANG Peng-gang. Chloride ions transport and adsorption in the nano-pores of silicate calcium hydrate: Experimental and molecular dynamics studies [J]. Construction and Building Materials, 2016, 126: 991-1001. DOI: 10.1016/ j.conbuildmat.2016.09.110.
[23] ZHOU Yang, TANG Lu-ping, LIU Jia-ping, MIAO Chang- wen. Interaction mechanisms between organic and inorganic phases in calcium silicate hydrates/poly(vinyl alcohol) composites [J]. Cement and Concrete Research, 2019, 125: 105891. DOI: 10.1016/j.cemconres.2019. 105891.
[24] ZHOU Yang, HOU Dong-shuai, JIANG Jin-yang, SHE Wei, LI Jia-qi. Molecular dynamics study of solvated aniline and ethylene glycol monomers confined in calcium silicate nanochannels: A case study of tobermorite [J]. Physical Chemistry Chemical Physics, 2017, 19(23): 15145-15159. DOI: 10.1039/ c7cp02928d.
[25] ZHOU Yang, HOU Dong-shuai, GENG Guo-qing, FENG Pan, YU Jiao, JIANG Jin-yang. Insights into the interfacial strengthening mechanisms of calcium-silicate-hydrate/ polymer nanocomposites [J]. Physical Chemistry Chemical Physics, 2018, 20(12): 8247-8266. DOI: 10.1039/c8cp0032 8a.
[26] WANG Hao, LIN En-qiang, XU Guang-ji. Molecular dynamics simulation of asphalt-aggregate interface adhesion strength with moisture effect [J]. International Journal of Pavement Engineering, 2017, 18(5): 414-423. DOI: 10.1080/10298436.2015.1095297.
[27] XU Guang-ji, WANG Hao. Molecular dynamics study of interfacial mechanical behavior between asphalt binder and mineral aggregate [J]. Construction and Building Materials, 2016, 121: 246-254. DOI: 10.1016/j.conbuildmat.2016.05. 167.
[28] GUO Qing-lin, LI Guang-yao, GAO Ying, WANG Ke-yi, DONG Zi-zhen, LIU Fu-chun, ZHU Han. Experimental investigation on bonding property of asphalt-aggregate interface under the actions of salt immersion and freeze-thaw cycles [J]. Construction and Building Materials, 2019, 206: 590-599. DOI: 10.1016/j.conbuildmat.2019.02.094.
[29] ZHOU Yang, HOU Dong-shuai, MANZANO H, OROZCO C A, GENG Guo-qing, MONTEIRO P J M, LIU Jia-ping. Interfacial connection mechanisms in calcium-silicate- hydrates/polymer nanocomposites: A molecular dynamics study [J]. ACS Applied Materials and Interfaces, 2017, 9(46): 41014-41025. DOI: 10.1021/acsami.7b12795.
[30] PICKER A, NICOLEAU L, NONAT A, LABBEZ C, COLFEN H. Identification of binding peptides on calcium silicate hydrate: A novel view on cement additives [J]. Advanced Materials, 2014, 26(7): 1135-1140. DOI: 10.1002/adma.201303345.
[31] GENG Guo-qing, MYERS R J, QOMI M J A, MONTEIRO P J M. Densification of the interlayer spacing governs the nanomechanical properties of calcium-silicate-hydrate [J]. Scientific Reports, 2017, 7(1): 10986. DOI: 10.1038/s41598- 017-11146-8.
[32] RICHARDSON I G. The calcium silicate hydrates [J]. Cement and Concrete Research, 2008, 38(2): 137-158. DOI: 10.1016/j.cemconres.2007.11.005.
[33] CONG X D, KIRKPATRICK R J, DIAMOND S. 29SI MAS NMR spectroscopic investigation of alkali-silica reaction product gels [J]. Cement and Concrete Research, 1993, 23(4): 811-823.
[34] KALINICHEV A G, KIRKPATRICK R J. Molecular dynamics modeling of chloride binding to the surfaces of calcium hydroxide, hydrated calcium aluminate, and calcium silicate phases [J]. Chemistry of Materials, 2002, 14(8): 3539-3549. DOI: 10.1021/cm0107070.
[35] DING Qing-jun, YANG Jun, HOU Dong-shuai, ZHANG Gao-zhan. Insight on the mechanism of sulfate attacking on the cement paste with granulated blast furnace slag: An experimental and molecular dynamics study [J]. Construction and Building Materials, 2018, 169: 601-611. DOI: 10.1016/j.conbuildmat.2018.02.148.
[36] ZHOU Yang, CAI Jing-shun, HOU Dong-shuai, CHANG Hong-lei, YU Jiao. The inhibiting effect and mechanisms of smart polymers on the transport of fluids throughout nano- channels [J]. Applied Surface Science, 2020, 500: 144019. DOI: 10.1016/j.apsusc.2019.144019.
[37] WANG Jian-wei, KALINICHEV A G, KIRKPATRICK R J. Molecular modeling of water structure in nano-pores between brucite (001) surfaces [J]. Geochimica et Cosmochimica Acta, 2004, 68(16): 3351-3365. DOI: 10.1016/j.gca.2004.02.016.
[38] WANG Jian-wei, KALINICHEV A G, KIRKPATRICK R J. Effects of substrate structure and composition on the structure, dynamics, and energetics of water at mineral surfaces: A molecular dynamics modeling study [J]. Geochimica et Cosmochimica Acta, 2006, 70(3): 562-582. DOI: 10.1016/j.gca.2005.10.006.
[39] MARTIN M, ESPINOSA J F, ASENSIO J L, JIMENEZ J. A comparison of the geometry and of the energy results obtained by application of different molecular mechanics force fields to methyl α-lactoside and the c-analogue of lactose [J]. Carbohydrate Research, 1997, 298(1, 2): 15-49. DOI: 10.1016/S0008-6215(96)00225-X.
[40] ASENSIO J L, MARTIN M, JIMENEZ J. The use of CVFF and CFF91 force fields in conformational analysis of carbohydrate molecules. comparison with AMBER molecular mechanics and dynamics calculations for methyl α-lactoside [J]. International Journal of Biological Macromolecules, 1995, 17(3, 4): 137-148. DOI: 10.1016/ 0141-8130(95)92680-O.
[41] SANCHEZ F, ZHANG L. Interaction energies, structure, and dynamics at functionalized graphitic structure-liquid phase interfaces in an aqueous calcium sulfate solution by molecular dynamics simulation [J]. Carbon, 2010, 48(4): 1210-1223. DOI: 10.1016/j.carbon.2009.11.044.
[42] XU Guang-ji, WANG Hao. Study of cohesion and adhesion properties of asphalt concrete with molecular dynamics simulation [J]. Computational Materials Science, 2016, 112: 161-169. DOI: 10.1016/j.commatsci.2015.10.024.
[43] SRDJAN K, JELENA B V, PAUL G T, GIJSBERTUS D W, COR E K. Estimating the polymer-metal work of adhesion from molecular dynamics simulations [J]. Chemistry of Materials, 2007, 19(4): 903-907. DOI: 10.1021/cm0621702.
[44] SHI Jia-jun, PAN Yun-feng, LI He-dong, FU Jun. Effects of water immersion on the adhesion between adhesive layer and concrete block [J]. Advances in Civil Engineering, 2019, 2019: 16-18. DOI: 10.1155/2019/7069757.
[45] BEUSHAUSEN Hans, HOHLIG Bjorn, TALOTTI Marco. The influence of substrate moisture preparation on bond strength of concrete overlays and the microstructure of the OTZ [J]. Cement and Concrete Research, 2017, 92: 84-91. DOI: 10.1016/j.cemconres.2016.11.017.
[46] JEBLI M, JAMIN F, PELISSOU C, MALACHANNE E, GARCIA-DIAZ E, EL YOUSSOUFI M S. Leaching effect on mechanical properties of cement-aggregate interface [J]. Cement and Concrete Composites, 2018, 87: 10-19. DOI: 10.1016/j.cemconcomp.2017.11.018.
[47] DONG Ze-jiao, LIU Zhi-yang, WANG Peng, GONG Xiang-bing. Nanostructure characterization of asphalt- aggregate interface through molecular dynamics simulation and atomic force microscopy [J]. Fuel, 2017, 189: 155-163. DOI: 10.1016/j.fuel.2016.10.077.
[48] ZHAO Hai-tao, JIANG Kai-di, YANG Rui, TANG Yi-min, LIU Jia-ping. Experimental and theoretical analysis on coupled effect of hydration, temperature and humidity in early-age cement-based materials [J]. International Journal of Heat and Mass Transfer, 2020, 146: 118784. DOI: 10.1016/ j.ijheatmasstransfer.2019.118784.
[49] ZHAO Hai-tao, WU Xia, HUANG Yu-yu, ZHANG Peng, TIAN Qian, LIU Jia-ping. Investigation of moisture transport in cement-based materials using low-field nuclear magnetic resonance imaging [J]. Magazine of Concrete Research, 2021, online. DOI: 10.1680/jmacr.19.00211.
[50] SANCHEZ F, ZHANG L. Molecular dynamics modeling of the interface between surface functionalized graphitic structures and calcium-silicate-hydrate: Interaction energies, structure, and dynamics [J]. Journal of Colloid and Interface Science, 2008, 323(2): 349-358. DOI: 10.1016/j.jcis.2008. 04.023.
[51] THOMAS R J, SORENSEN A D. Review of strain rate effects for UHPC in tension [J]. Construction and Building Materials, 2017, 153: 846-856. DOI: 10.1016/ j.conbuildmat.2017.07.168.
[52] WU Sheng-xing, CHEN Xu-dong, ZHOU Ji-kai. Influence of strain rate and water content on mechanical behavior of dam concrete [J]. Construction and Building Materials, 2012, 36: 448-457. DOI: 10.1016/j.conbuildmat.2012.06.046.
[53] WU Sheng-xing, CHEN Xu-dong, ZHOU Ji-kai. Tensile strength of concrete under static and intermediate strain rates: Correlated results from different testing methods [J]. Nuclear Engineering and Design, 2012, 250: 173-183. DOI: 10.1016/ j.nucengdes.2012.05.004.
(Edited by FANG Jing-hua)
中文导读
水化硅酸钙-集料界面交互作用及湿度影响的分子动力学研究
摘要:水泥水化浆体与集料的界面性质在很大程度上决定了混凝土的各项性能。本研究利用分子动力学模拟探讨了常用的集料相碳酸钙/二氧化硅与水化硅酸钙(C-S-H)之间的界面相互作用机制,以及湿度对界面性质的影响。结果表明,无论在干或湿条件下,C-S-H/碳酸钙界面都具有较强的稳定性。这是由于碳酸钙中钙离子与C-S-H表面的高极性非桥氧原子具有高强度化学连接的原因。二氧化硅则通过氢键作用吸附于干燥的C-S-H表面,但界面上水分子的存在会大大降低界面亲和度。此外,还利用分子动力学进行了C-S-H/集料界面分离的动态模拟,计算结果趋势符合准静态黏聚法则。而润湿度和拉伸速率对C-S-H/二氧化硅的分离过程有较为明显的影响,较湿润的界面和较小的加载速率均有可能导致黏聚力降低。本研究可为纳米尺度下理解浆体/集料相互作用以及高性能水泥基材料的研制提供新的思路。
关键词:水化硅酸钙;集料;界面连接;分子动力学模拟;湿度
Foundation item: Projects(6512009004A, 51908119, U1706222) supported by the National Natural Science Foundation of China; Project(BK20190367) supported by the Natural Science Foundation of Jiangsu Province, China
Received date: 2020-03-15; Accepted date: 2020-07-24
Corresponding author: HUANG Jia-le, PhD Candidate; Tel: +86-25-52090672; E-mail: jialehuang@seu.edu.cn; ORCID: https://orcid.org/ 0000-0001-7135-8577

