
Leaching of low grade zinc oxide ores in Ida2--H2O system
DOU Ai-chun, YANG Tian-zu, YANG Ji-xing, WU Jiang-hua, WANG An
School of Metallurgical Science and Engineering, Central South University. Changsha 410083, China
Received 31 March 2010; accepted 2 June 2011
Abstract: Ida2--H2O system (iminodiacetate aqueous solution) was used to leach a low grade zinc oxide ore for Zn extraction. The effects of leaching time, liquid-solid ratio (L/S), total concentration of Ida2- ([Ida2-]T), leaching temperature and pH on Zn leaching recovery and the dissolution of impurities such as Ca, Mg, Cu, Ni, Fe, Pb and Cd were investigated. Results show that Ca, Mg and Fe in ores were hardly dissolved in alkalescent iminodiacetate aqueous solution, while valuable metals such as Cu, Ni, Pb and Cd were partly dissolved into leaching liquor with Zn. The recovery of Zn reaches 76.6% when the ores were leached for 4 h at 70 °C by 0.9 mol/L iminodiacetate aqueous solution with pH of 8 and L/S of 5:1.
Key words: Ida2-; zinc oxide ore; leaching
1 Introduction
Nowadays, with the depletion of zinc sulphuric ores which are easily upgraded by floatation, low grade zinc oxide ores, which are difficult to be upgraded and smelt, have drawn more and more attention. In our country, the resource of zinc oxide ores with low grade of zinc, high content of gangue and complicated phase composition are very abundant. Therefore, it is of great strategic significance to effectively utilize these low grade zinc oxide ores.
Both pyrometallurgical and hydrometallurgical methods [1-2] had been employed to treat low grade zinc oxide ores. But now, the pyrometallurgical methods [3] have been replaced by hydrometallurgical methods for its low recovery of Zn with high energy consumption and hazard pollution. Hydrometallurgical treatment mainly contains acid leaching process, ammonia leaching process and alkaline leaching process. Acid leaching [4-5] is a non-selective system for valuable metals’ dissolution due to that lixiviant is H2SO4 mostly and the impurities such as Ca, Mg, Fe and Si in ores can be dissolved in acidic solutions during leaching process, which results in more consumption of leaching agent and the leaching liquor contaminated. What’s worse, Si easily forms silica gels in acidic media which will affect solid-liquid separation consequently.
Ammonia leaching and alkaline leaching are the selective system for Zn extraction by the formation of complexes. In ammonia leaching, NH3, serving as the main ligand, can complex with Zn2+ and convert it into solution with little amount of Ca, Mg, Fe and Si dissolution. However, ammonia in the system evaporates easily, which causes the environment pollution. Current studies [6-8] about ammonia leaching still focus on utilizing ammonium to substitute part of ammonia to alleviate the environmental problem.
OH- is also used as the leaching ligand to coordinate with Zn2+ for Zn extraction from ores in alkaline leaching [9-11]. It is a challenge for facilities to resist the corrosion of strong basic system in operation. Moreover, SiO2 will be dissolved in leaching liquor as silicates under so high concentration of OH- in the process.
Recent years, many researches [12-16] have been done about ammonia leaching and alkaline leaching to deal with low grade zinc oxide ores. Good techno-economic indicators in operation have been obtained in these two processes, but ligands were only limited to NH3, OH- and Cl-. In order to get some new ligands more appropriate to leach these low grade zinc oxide ores, some work had been done [17] about ligand selection for complex-leaching valuable metals in hydrometallurgy. The results showed that ligand of iminodiacetate (Ida2-) could be used to leach zinc oxide ores in aqueous solution at pH 8-11. According to this result, experiments of a low grade zinc oxide ore leached by Ida2--H2O system were done in the present work, and the dissolution of impurities in this leaching system was also studied.
2 Experimental
2.1 Materials
The zinc oxide ores used in the present study was from Lanping town, Yunnan province of China. After being crashed and ground, more than 95% of the ore particles have a size less than 150 μm, and the corresponding major components and mineralogical compositions are listed in Table 1 and Table 2, respectively. The tables show that the ore has a low zinc content with major phase of smithsonite (ZnCO3 85.08% in non-sulphide zinc), a high content of gangue (basic gangue w(CaO)+w(MgO)>25%, acid gangue w(SiO2) 17.53%), and 6.5% of w(Fe). Actually, other phases of zinc oxides such as hemimorphite (Zn4Si2O7(OH)2·H2O), hydrozincite (Zn5(CO3)2(OH)6) and zincite (ZnO) can also be dissolved in weak basic system of Ida2--H2O, while SiO2 cannot be.
Table 1 Chemical composition of low grade zinc oxide ores (mass fraction, %)

Table 2 Phase composition of Zn in low grade zinc oxide ores (mass fraction, %)

2.2 Procedure and analysis
Experiments were carried out in a beaker of 250 mL which was heated in a constant temperature water bath with a stirring speed of 300 r/min. According to the desired liquid to solid ratio (L/S), 20 g ore was added to the Ida2--H2O system with required Ida2- concentration ([Ida2-]T), temperature and pH value respectively, where the concentration of Ida2- was adjusted by solid IDA (AR) and pH controlled by solid NaOH (AR). When the leaching process finished in a required time interval, leaching liquor and washing water were collected to measure their metal contents.
The concentration of Zn in aqueous was analyzed by EDTA volumetric method. Especially, since the complex affinity of Ida2- with Zn2+ is stronger than EDTA, Zn analysis in solution can only be carried out after Zn2+-Ida2- complexes being destroyed by adding concentrated sulphuric acid. Impurities in leaching liquor were analyzed by atomic absorption spectroscopy (AAS). Impurities (concentration) in different volume of each sample were calculated in a uniform volume for investigating dissolution conveniently.
3 Results and discussion
3.1 Effect of leaching time
The recovery of Zn in the ore and the dissolution of impurities in leaching liquor were examined against leaching time under the condition of 0.9 mol/L [Ida2-]T, leaching temperature 70 °C, L/S 5:1 and pH 9. The results plotted in Fig. 1 indicate that leaching time had little effect on Zn recovery, which only rose to less than 4% when time lasted from 1 h to 6 h. The recovery of Zn kept a little invariable when ores were leached for more than 4 h. Meanwhile, data in Table 3 show that leaching time nearly had no effect on the dissolution of impurities in leaching liquor. Therefore, leaching time should be controlled in 4 h for a lower energy consume.
In Table 3, alkali gangue (CaO+MgO) and Fe with high content in ore had low dissolution in Ida2--H2O system under the above conditions. Valuable metals Pb, Cd, Cu and Ni which were concomitant with Zn of low content in the ore had partial dissolution into leach liquor, which makes it more important for comprehensive recovery of valuable metals in low grade zinc oxide ores.

Fig. 1 Effect of leaching time on Zn recovery
Table 3 Effect of leaching time on dissolution of impurities in leaching liquor

3.2 Effect of L/S
Under the conditions of 0.9 mol/L [Ida2-]T and pH 9, the ores were leached at different L/S (3-7) for 4 h at 70 °C in Ida2--H2O system. Recovery of Zn in Fig. 2 increased obviously from 66.06% to 72.69% with the increase of L/S from 3 to 5. When L/S was more than 5:1, Zn leaching recovery changed slowly. Table 4 shows that L/S had no effect on the dissolution of Mg, Ni, Cu and Cd, while the concentration of Pb and Ca increased slowly with increasing the L/S. Considering that the highest concentrations of Pb and Ca were only 426.61 mg/L and 176.06 mg/L respectively, L/S of 5:1 may be optimal.
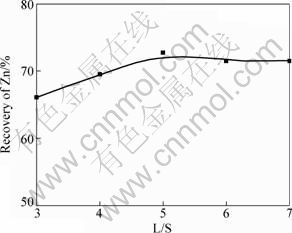
Fig. 2 Effect of liquid-solid ratio on Zn recovery
3.3 Effect of [Ida2-]T
Leaching recovery of Zn in Fig. 3 and the dissolution of impurities in Table 5 were plotted against the total concentration of Ida2- under the conditions of leaching zinc oxide ores for 4 h at 70 °C with pH 9 and L/S 5:1 in the Ida2--H2O system. The complexation of Ida2- with metallic ions were improved with the increase of [Ida2-]T in the system, and the dissolution of Zn and impurities increased in leaching liquor correspondingly. When [Ida2-]T increased from 0.5 mol/L to 0.9 mol/L, leaching recovery of Zn rose from 67.8% to 71.89%. While [Ida2-]T was more than 0.9 mol/L, recovery of Zn changed slightly. In Table 5, dissolution of impurities in leaching liquor except for Cu and Ni had increased to some extent with [Ida2-]T growing. Changes of Cu and Ni were due to their low abundance in the ore and [Ida2-]T of 0.5 mol/L was adequate for their dissolution. Ca dissolved in the system increased rapidly with the growth of [Ida2-]T. In order to restrict the dissolution of Ca and enhance the leaching recovery of Zn, total concentration of Ida2- of 0.9 mol/L was optimal.

Fig. 3 Effect of total concentration of Ida2- on Zn recovery
Table 4 Effect of L/S on dissolution of impurities in leaching liquor

Table 5 Effect of [Ida2-]T on dissolution of impurities in leaching liquor

3.4 Effects of temperature and pH
When temperature changed from 25 °C to 85 °C, the ores were leached at different pH values for 4 h with L/S of 5:1 and [Ida2-]T of 0.9 mol/L in Ida2--H2O system. Leaching recovery of Zn and dissolution of impurities at various temperatures are given in Fig. 4 and Table 6, respectively. These results indicate that leaching temperature and pH had a significant effect on the dissolution of Zn and impurities in the system.
It seemed that there is nearly no formula in the graphs of Zn leaching recovery under different temperatures. But in some certain area, there are also some similar rules among the figures under several temperatures except for 25 °C and 85 °C. Leaching recovery of Zn went up with the increase of pH under 25 °C, while that reversed on graph of 85 °C. For other graphs of 40-70 °C, they all had areas where Zn leaching recovery increased with the increase of pH, and Zn leaching recovery decreased with the increase of pH, therefore a peak of Zn leaching recovery can be obtained. Interestingly, the peak of Zn leaching recovery emerged around pH of 11, 10, 9, 8 and 7 sequentially on graphs of 25 °C to 85 °C, which cannot be explained by kinetics. The reasons might be that the protonation of Ida2- with H+ was weakened with the increase of temperature in Ida2--H2O system, which caused more free ligand of Ida2- be released by IDA at a higher temperature. More free ligand of Ida2- at the higher temperature facilitating the complexation of Ida2- with Zn2+ happened in a lower pH.

Fig. 4 Effects of pH and temperature on Zn recovery
Table 6 Effects of temperature and pH on dissolution of impurities in leaching liquor

Moreover, Zn leaching recovery decreased rapidly at 85 °C with the increase of pH, which seems that a precipitation of Zn had been produced in the higher pH in the system. Reference [17] reported the decreasing tendency of [Zn2+]T (total concentration of Zn2+) at higher pH in Ida2--H2O system in thermodynamics. In order to verify this interpretation, some experiments were conducted as follows. 50 mL leaching liquor (obtained under condition: 20 g ore, 70 °C, pH 8, L/S 5:1 and [Ida2-]T 0.9 mol/L) was used. Firstly, its pH was adjusted by NaOH at 85 °C, then a precipitation emerged at pH 9.5, solid-liquid separation was not taken out until the pH reached 10. The precipitation was analyzed by XRD (Fig. 5) after washing and atmospheric drying. The result shows that the precipitation is ZnO. After precipitation, the concentration of Zn decreased from 10 g/L to 2.2 g/L, and 78% Zn had been deposited from leaching liquor. The verification test provided an idea for recovering of Zn from leaching liquor.
Data in Table 6 show that pH had greater effects on the dissolution of impurities in leaching liquor than temperature. Concentrations of Mg, Fe, Pb and Ca in leaching liquor declined with the increase of pH. The dissolution rule of Cd was similar to that of Zn, both of them decreased with the increase of pH at 85 °C. Concentrations of Cu and Ni in leaching liquor were stable because of their trace contents in ores. Actually, during precipitating Zn as ZnO at higher temperature and higher pH from leaching liquor, Cu and Ni can also be deposited by absorptive coprecipitation with oxides or hydroxides of Mg, Fe, Pb and Ca in residue of ZnO.
For enhancing the leaching recovery of Zn and restricting the dissolution of Ca, temperature 70 °C and pH 8 might be optimal.

Fig. 5 X-ray diffraction pattern of precipitation
4 Conclusions
1) Leaching temperature and pH have large effects on leaching recovery of Zn.
2) High content of basic gangue, acid gangue and Fe in low grade zinc oxide ores are hardly dissolved in weak basic Ida2--H2O system. Valuable metals Cu, Ni, Pb and Cd in ores are partially dissolved with Zn into leaching liquor.
3) When 20 g ore is leached for 4 h under 70 °C in the presence of 0.9 mol/L iminodiacetate aqueous solution with pH 8 and L/S 5:1, the leaching recovery of Zn reaches 76.6%.
4) Zn can be deposited as ZnO at higher temperature and higher pH in zinc-contained Ida2--H2O system, which helps for recovering Zn from leaching liquor.
References
[1] JHA M K, KUMAR V, SINGH R J. Review of hydrometallurgical recovery of zinc from industrial wastes [J]. Resources, Conservation and Recycling, 2001, 33(1): 1-22.
[2] FRENAY J. Leaching of oxidized zinc ores in various media [J]. Hydrometallurgy, 1985, 15(2): 243-253.
[3] NORGATE T, JAHANSHAHI S. Low grade ores—Smelt, leach or concentrate? [J]. Minerals Engineering, 2010, 23(2): 65-73.
[4] SOUZA A D, PINA P S, SANTOS F M F, DA SILVA C A, LE?O V A. Effect of iron in zinc silicate concentrate on leaching with sulphuric acid [J]. Hydrometallurgy, 2009, 95(3-4): 207-214.
[5] QIN Wen-qing, LI Wei-zhong, LAN Zhuo-yue. Simulated small-scale pilot plant heap leaching of low-grade oxide zinc ore with integrated selective extraction of zinc [J]. Minerals Engineering, 2007, 20(7): 694-700.
[6] JU Shao-hua, TANG Mo-tang, YANG Sheng-hai. Dissolution kinetics of smithsonite ore in ammonium chloride solution [J]. Hydrometallurgy, 2005, 80(1-2): 67-74.
[7] WANG Rui-xiang, TANG Mo-tang, LIU Wei, YANG Sheng-hai, ZHANG Wen-hai. Leaching of low grade zinc oxide ore in NH3-NH4Cl-H2O system [J]. The Chinese Journal of Process Engineering, 2008, 8(Supp.1): 219-222. (in Chinese)
[8] YANG Sheng-hai, TANG Mo-tang. Thermodynamics of Zn(Ⅱ)-NH3-NH4Cl-H2O system [J]. Transactions of Nonferrous Metals Society of China, 2000, 10(6): 830-833.
[9] YUAN Tie-chui, CAO Qin-yuan, LI Jie. Effects of mechanical activation on physicochemical properties and alkaline leaching of hemimorphite [J]. Hydrometallurgy, 2010, 104(2): 136-141.
[10] ZHAO Y C, ROBERT S. Production of Zn powder by alkaline treatment of smithsonite Zn-Pb ores [J]. Hydrometallurgy, 2000, 56(2): 237-249.
[11] FENG Lin-yong, YANG Xian-wan, SHEN Qing-feng. Pelletizing an alkaline leaching powdery low grade zinc oxide ores [J]. Hydrometallurgy, 2007, 89(3-4): 305-310.
[12] YIN Zhou-lan, DING Zhi-ying, HU Hui-ping, LIU Kui, CHEN Qi-yuan. Dissolution of zinc silicate (hemimorphite) with ammonia-ammonium chloride solution [J]. Hydrometallurgy, 2010, 103(1-4): 215-220.
[13] YIN Zhou-lan, DING Zhi-ying, HU Hui-ping, CHEN Qi-yuan. Dissolution kinetics of zinc silicate (hemimorphite) in ammoniacal solution [J]. Hydrometallurgy, 2010, 104(2): 201-206.
[14] CHEN Ai-liang, ZHAO Zhong-wei, JIA Xi-jun, LONG Shuang, HUOL Guang-sheng, CHENG Xing-yu. Alkaline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore [J]. Hydrometallurgy, 2009, 97(3-4): 228-232.
[15] WANG Rui-xiang, TANG Mo-tang, YANG Sheng-hai, ZHANG Wen-hai, TANG Chao-bo, HE Jing, YANG Jian-guang. Leaching kinetics of low grade zinc oxide ore in NH3-NH4Cl-H2O system [J]. Journal of Central South University of Technology, 2008, 15(5): 679-683.
[16] TANG Mo-tang, ZHANG Jia-liang, WANG Bo, YANG Sheng-hai, HE Jing, TANG Chao-bo, YANG Jian-guang. Cycle leaching of low grade zinc oxide ores in MACA system [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(1): 214-219. (in Chinese)
[17] YANG Tian-zu, DOU Ai-chun, LEI Cun-mao, REN Jin, LIU Zhen-zhen. Ligand selection for complex-leaching valuable metals in hydrometallurgy [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(6): 1148-1153.
Ida2--H2O体系浸出低品位氧化锌矿
窦爱春,杨天足,杨际幸,吴江华,王 安
中南大学 冶金科学与工程学院,长沙 410083
摘 要:采用Ida2--H2O体系(亚氨二乙酸盐水溶液)处理高碱性脉石型低品位氧化锌矿,考察浸出时间、液固比、配体总浓度、温度及pH值对矿物中主金属Zn及杂质元素Ca、Mg、Cu、Ni、Fe、Pb、Cd的溶出影响。结果表明:在弱碱性Ida2--H2O体系中,Ca、Mg、Fe不会被大量溶出,有价金属Cu、Ni、Pb、Cd可部分随主金属Zn溶出而进入浸出液;在浸出时间4 h、液固比5:1、配体总浓度0.9 mol/L、温度70 °C、pH8的优化条件下,锌浸出率为76.6%。
关键词:Ida2-;氧化锌矿;浸出
(Edited by YANG Hua)
Foundation item: Project (2007CB613604) supported by the National Basic Research Program of China
Corresponding author: YANG Tian-zu; Tel: +86-731-88836791; E-mail: tianzuyang@163.com
DOI: 10.1016/S1003-6326(11)61049-2