镁合金AZ31无铬无氟无亚硝酸盐磷化膜的制备及耐蚀性能
来源期刊:中国有色金属学报(英文版)2012年第11期
论文作者:崔学军 刘春海 杨瑞嵩 李明田 林修洲 龚 敏
文章页码:2713 - 2718
关键词:镁合金;AZ31合金;磷酸盐转化膜;无铬;耐蚀;阳极溶解;阴极析氢
Key words:magnesium alloy; AZ31 alloy; phosphate conversion coating; chromate-free film; corrosion resistance; anodic dissolution; cathodic hydrogen evolution
摘 要:以磷酸二氢锰和无氟、无铬、无亚硝酸盐的添加剂为主要成分,通过化学沉积的方法在镁合金AZ31表面获得致密均匀的耐蚀磷化膜。通过硫酸铜点蚀测试、SEM、XRD及电化学极化曲线等表征手段,详细研究了膜层的形貌、组成、相结构及耐蚀性能,讨论了成膜温度和游离酸对膜层微结构、形貌及耐蚀性能的影响。结果表明,磷化膜通过抑制阳极溶解和阴极析氢,有效地提高了镁合金AZ31的耐蚀性能。
Abstract: A phosphate solution free of chromate, fluoride and nitrite was prepared and an environment-friendly film was obtained on AZ31 magnesium alloy surface via the chemical deposition method. The morphology, composition, phase structure and its corrosion resistance were studied. The effects of film-forming temperature and free acid on corrosion resistance, microstructure and electrochemical behavior of the film were discussed. The results indicate that the corrosion resistance of AZ31 with the phosphate film was better than blank AZ31 substrate, which was most attributed to the great inhibitive action on the anodic dissolution and cathodic hydrogen evolution of the film.
Trans. Nonferrous Met. Soc. China 22(2012) 2713-2718
CUI Xue-jun1, 2, LIU Chun-hai1, 2, YANG Rui-song1, 2, LI Ming-tian1, 2, LIN Xiu-zhou1, 2, GONG Min1, 2
1. Material Corrosion and Protection Key Laboratory of Sichuan Province, Sichuan University of Science and Engineering, Zigong 643000, China;
2. College of Materials and Chemical Engineering, Sichuan University of Science and Engineering, Zigong 643000, China
Received 8 August 2011; accepted 16 February 2012
Abstract: A phosphate solution free of chromate, fluoride and nitrite was prepared and an environment-friendly film was obtained on AZ31 magnesium alloy surface via the chemical deposition method. The morphology, composition, phase structure and its corrosion resistance were studied. The effects of film-forming temperature and free acid on corrosion resistance, microstructure and electrochemical behavior of the film were discussed. The results indicate that the corrosion resistance of AZ31 with the phosphate film was better than blank AZ31 substrate, which was most attributed to the great inhibitive action on the anodic dissolution and cathodic hydrogen evolution of the film.
Key words: magnesium alloy; AZ31 alloy; phosphate conversion coating; chromate-free film; corrosion resistance; anodic dissolution; cathodic hydrogen evolution
1 Introduction
Magnesium alloys have many outstanding physical and mechanical properties such as low density, high specific strength, good castability, good weldability and machinability [1], which make them ideal candidates for light-weight engineering applications in the fields, for instance, automobile, electronic, aerospace and railway industries. However, the poor corrosion resistance of magnesium alloys restricts their use on a larger scale. Particularly, the corrosion resistance of AZ31 magnesium alloy is relatively lower compared with that in common AZ91 and AM60 magnesium alloys [2,3]. In order to improve the corrosion resistance of AZ31 magnesium alloy, many methods have been developed, including the low cost chemical conversion film method where chromate bath is used [4,5]. It is worth noting that hexavalent chromium ions in the chromate bath are toxic [6] and thus various novel environmental friendly conversion coatings have been reported, such as the phytic acid conversion coatings [7], oxalate coatings [8], cerium conversion coatings [9], stannate conversion coatings [4], silane films [10] and phosphate coatings [5]. However, the coatings obtained from the above mentioned methods are generally thin and of poor corrosion resistance except for phosphate coatings. The phosphate coatings are of good corrosion resistance and self healing performances, but the conventional phosphate solutions [5,11] contain many hazardous substances such as fluorides, nitrites and heavy metal elements. Therefore, it is essential to develop a novel environmental friendly anticorrosive coating for the AZ31 magnesium alloy. ISHIZAKI et al [12] reported that a novel anticorrosive film was obtained on the surface of AZ31 magnesium alloy through a mixed aqueous solution containing H3PO4 and α-Ca3(PO4)2, and no toxic elements such as chromium, fluorine and nitrogen were used in the method. SONG et al [13] also obtained a novel phosphate conversion film on the magnesium alloy surface through an environment- friendly solution containing Ca(NO3)2 and NH4H2PO4. Thus, to prepare corrosion resistant phosphate films on the magnesium alloy surface is a good method through decreasing or no using harmful ingredients in the solution.
In this work, an environmental friendly phosphate solution free of chromate, fluorides and nitrite was prepared. We will show that the corrosion resistant conversion film can be fabricated on the AZ31 magnesium alloy surface through this phosphate solution, which will enhance the corrosion resistance of the AZ31 magnesium alloy. Moreover, the morphologies, compositions, phase structures of the films and the corrosion resistance were studied. The effects of film-forming temperature and free acid on corrosion resistance, microstructure and electrochemical behavior of the film were also discussed.
2 Experimental
The substrate material was AZ31 magnesium alloy (composition in mass fraction: 2.96% Al, 0.89% Zn, 0.37% Mn, 0.0134% Si, 0.001% Cu, 0.002% Ni, 0.0027% Fe, with the remainder being Mg, thickness of 2.0 mm, supplied by a company). Before conversion treatment, the pretreatments of the substrate were carried out and listed as follows: abrading (water-proof abrasive papers of 300-500 grade) → being in distilled water bath → alkali washing (60%NaOH) → being in distilled water bath → acid activation (75%H3PO4) → being in distilled water bath → surface conditioning → phosphating → drying (120 °C, 20 min).
All chemicals were of reagent grade and used without further purification. The compositions of the phosphate solution were chosen according to Refs. [14,15]. The phosphate solution was mainly composed of 40 g/L dihydrogen phosphate manganese (Mn(H2PO4)2), 0.540 g/L polyphosphate, and the pH of the solution was adjusted in the range of 3.0-6.0 by H3PO4 or NaOH. In order to prepare the corrosion-resistant conversion film, the AZ31 magnesium alloy samples were treated at 40-95 °C for 25 min in the phosphate solution.
The corrosion resistance time of the film was tested through a CuSO4 pitting corrosion method according to GB 6807—86. The surface morphologies, cross-sectional view and the compositions of the obtained films were studied using scanning electron microscope (SEM, VEGA 3 SBU, Czekh) with energy dispersive spectrometer (EDS, England Oxford) and X-ray diffractometer with Cu Kα radiation (XRD, Japan Rigaku, D/max-rB).
The neutral salt spray test (NSS) was adopted according to ASTM B117—07 to evaluate the pitting corrosion resistances of blank and coated AZ31 alloy. The test was carried out under continuous spray conditions at 35±2 °C. The samples were inspected every 12 h. For the painted samples, before salt spray tests, two crossed beelines on the surface of the samples were scored using a knife (ISO2409). The nick must be scored to the substrate. The adhesion tests of the painted samples were conducted according to ISO2409 standard, which is a test method for assessing the resistance of paint coatings to separation from substrates when a right angle lattice pattern is cut onto the coating, penetrating to the substrate. Finally, the macroscopic surface appearances were obtained through digital camera with 8.0 mega pixels.
All the electrochemical experiments were carried out in a 3.5% NaCl solution at room temperature on the electrochemical system (PARSTAT 2273, America). The test instrument was composed of three-electrode system. A saturated calomel electrode (SCE) was used as the reference electrode. The platinum sheet with a surface area of 1 cm2 was used as the auxiliary electrode. The sample with a surface area of about 1 cm2 was the working electrode. The potentiodynamic electrochemical tests were carried out with a scan rate of 1 mV/s. The standing time was 20 s, and corrosion inhibition efficiency η=100×(1-R0P/RP), where R0P and RP are the average polarization resistances of the AZ31 and AZ31 plus film, respectively. The corrosion parameters were calculated via self-soft in the computer.
3 Results
3.1 Morphologies and crystalline structure
Figure 1 shows the SEM and EDS of the prepared film on AZ31 magnesium alloy surface. The film is gray black and the surface is very dense, free of cracks. Surprisingly, the film consists of many big particles with more sheets of a few micron thickness (Figs. 1(a) and (b)). The morphology of the interface between the film and AZ31 alloy is shown in Fig. 1(c) where good bonding of the film and substrate can be observed. Figure 1(d) displays the EDS analysis of the elements around the interface of the phosphate film and the substrate was conducted by line scanning. The compositions of the phosphate film are mainly composed of O, Mg, P, Mn and Al (Fig. 1(d)), and the peaks assigned to Mg and Al are ascribed to the AZ31 magnesium alloy. No other toxic element such as chromium, fluorine and nitrogen can be found, which means that the film is environmental friendly.
Figure 2 presents XRD pattern of the AZ31 magnesium alloy with phosphate film. The main diffraction peaks observed in Fig. 2 can be assigned to those of MnHPO4·2.25H2O, indicating that the film is mainly composed of the phosphates of manganese, no other diffraction peaks of chromate, fluoride and nitrate, in agreement with the results of EDS.
3.2 Neutral salt spry test
Figure 3 shows the macroscopic surface appearances of AZ31 magnesium alloy in different surface states after neutral salt spray test. Many white corrosion products and pits can be observed on the surface of blank AZ31 alloy without phosphate film after being tested under salt spray for 12 h, as shown in Fig. 3(a). However, good quality can be observed in Fig. 3(b) where the salt spray test was carried out for 72 h on the surface of the AZ31 alloy with phosphate film and the time of the salt spray is five times larger than that for the blank AZ31 alloy. To further prove the good corrosion resistance of the phosphate film, the AZ31 alloys with paint were checked in the neutral spray test. Figure 3(c) shows the morphology of the surface of the AZ31 alloy with paint after being tested for 24 h where the surface is severely corroded. However, the quality of the surface of the AZ31 alloy with paint and phosphate film is very good, as shown in Fig. 3(d). These results indicate that corrosion resistance of the AZ31 alloy is enhanced by using the phosphate film, and also further to prove that the insoluble phosphates can effectively improve the corrosion resistance of the AZ31 magnesium alloy [12]. Moreover, good adhesion with paint is also observed from the lattice pattern of the surface (Fig. 2(d)), which could meet the adhesion demand of automobile components [11].
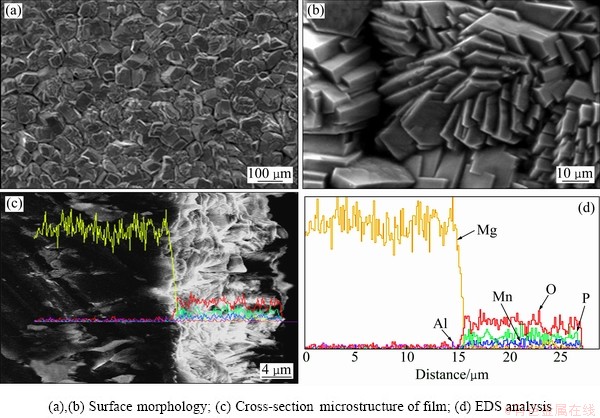
Fig. 1 SEM images (a,b,c) and EDS (d) of phosphate film on AZ31 magnesium alloy
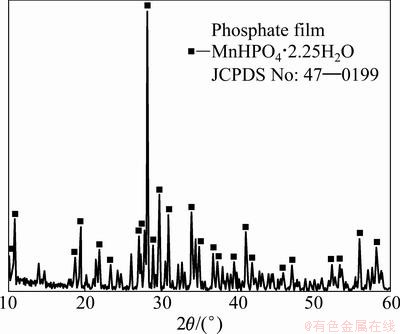
Fig. 2 XRD pattern of AZ31 magnesium alloy with phosphate film
4 Discussion
4.1 Effect of film-forming temperature on corrosion resistance and microstructure
The film-forming temperature is one of the most important factors on corrosion resistance of the phosphate film [16]. Therefore, the effect of film- forming temperature on time of CuSO4 pitting corrosion test of phosphate film and corresponding surface morphologies at 75, 85 and 95 °C are shown in Fig. 4. The CuSO4 pitting corrosion time is very short when the film-forming temperature is lower than 60 °C. However, the time increases rapidly when the forming temperature is higher than 60 °C. The trends indicate that the corrosion resistance will be improved gradually with the increasing of the film-forming temperature. The SEM image of the film formed at 75 °C shows that the compactness of the film is not good, the particle shapes are elliptic, the average size is about 20 μm, and the substrate can be clearly seen at 75 °C. The compactness of the film is improved when the phosphate film is formed at 85 °C. Moreover, the shapes of grains are not elliptic, but lamellar. This structure can improve the corrosion resistance of the film due to the increasing of the channel of corrosion substances [17]. The fine grains can be observed and the densest phosphate film is obtained at 95 °C. The concentrations of the ions in the phosphate solution have many effects on the properties of phosphate film and the service cycle of phosphate solution, which can be controlled by the film-forming temperature. The film-forming ion concentration cannot meet ionic product when the temperature is low, which will not obtain perfect and dense phosphate film at 75 °C. Therefore, with increasing the film-forming temperature, the grains can be refined and the film becomes denser, and the CuSO4 pitting corrosion time increases.

Fig. 3 Macroscopic surface appearances of AZ31 magnesium alloy in different surface states after neutral salt spray test for different time

Fig. 4 Effects of film-forming temperature on time of CuSO4 pitting corrosion test for phosphate film (a) and corresponding surface morphologies at 75 °C (b), 85 °C (c) and 95 °C (d)
4.2 Effects of free acid on corrosion resistance
Figure 5 shows the effect of free acid (FA) on time of CuSO4 pitting corrosion test of the phosphate film. The time is lower than 200 s when the FA is less than 2.0, and the time increases to about 500 s when the FA is about 4.0, and then the time begins to decrease gradually with further increasing the FA. In general, the phosphate solution is unstable and easy to produce additional residues when the FA content is too low. On the contrary, the over high FA will accelerate the substrate dissolution, which is unfavorable to form phosphate film. Therefore, the time of CuSO4 pitting corrosion test of phosphate film increases at first, then decreases with further increasing FA. When the film-forming temperature is over high, the dissociation degree of the soluble phosphate will increase, which can lead to dramatic increasing of the concentration of the film-forming ions and plenty of residues phosphide [16]. So, free acid (FA) was used to control the dissociation degree of the soluble phosphate and the concentration of the film-forming ions. The analysis results indicate that the optimum range of the FA is from 3.5 to 4.5.

Fig. 5 Effect of FA on time of CuSO4 pitting corrosion test of phosphate film (Error bars: 5%)
4.3 Electrochemical behavior
Corrosion current density (Jcorr), corrosion potential (φcorr) and polarization resistance (Rp) are often used to evaluate the corrosion protective property of the coatings [18]. Figure 6 shows the potentiodynamic polarization curves tested in 3.5% NaCl (mass fraction) solution for the blank AZ31 magnesium alloy and the phosphate samples at different film-forming temperatures. The corrosion parameters were calculated and listed in Table 1. Although the pitting potential of magnesium alloys is normally more negative than its corrosion potential in a chloride containing solution [2], the influence of film-temperature on the anodic polarization appears to be clear in this study. So, the corrosion rate of the AZ31 magnesium alloy can be estimated from its polarization curve according to Tafel extrapolation. The φcorr, Jcorr and Rp of the AZ31 magnesium alloy are -1566 mV (vs SCE), 5.02×10-4 A/cm2 and 69 Ω·cm2, respectively, which are in accordance with Refs. [3] and [12]. Compared with the phosphating AZ31 magnesium alloy, the φcorr and Rp are lower and the Jcorr is considerably higher, which implies that the phosphate film has much higher corrosion resistance than the blank AZ31. Moreover, the corrosion resistance property is enhanced gradually with the increasing film-forming temperature. When the film-forming temperature is 95 °C, the φcorr with the film is shifted positively by 102 mV (vs SCE), the Jcorr decreases approximately by four magnitude orders and the η reaches 99.99% compared with the blank substrate. The results state that the corrosion rate becomes slower and the corrosion gets more difficult. This clearly indicates that the phosphate film has a great inhibitive action on anodic dissolution. Moreover, with increasing the film-forming temperature, the inhibitive role of the film is more and more prominent. Nevertheless, the cathodic polarization curves still reliably represent the catholic hydrogen evolution activity [2]. The trend with the film-forming temperature is the same as its anodic polarization curve. The phosphating samples are in the group of lower cathodic polarization current density than the blank magnesium alloy AZ31. The results also show that the phosphate can effectively inhibit the cathodic hydrogen evolution. Therefore, the good corrosion resistance of the AZ31 magnesium alloy with the film can be attributed to great inhibitive action on the anodic dissolution and restraint action on the cathodic hydrogen evolution of the phosphate film.

Fig. 6 Potentiodynamic polarization curves tested in 3.5% NaCl solution for blank AZ31 magnesium alloy and phosphate samples at different film-forming temperatures
Table 1 Relevant electrochemical parameters of potentiodynamic polarization curves calculated from Tafel plot

5 Conclusions
1) An environmental friendly phosphate solution free of chromate, fluorides and nitrite was prepared and a high corrosion-resistant film was obtained on the AZ31 magnesium alloy surface from this solution. The uniform and compact phosphate films formed are mainly composed of MnHPO4 ·2.25H2 O, the film thickness is between 12 μm and 15 μm and the composition consists of O, Mg, P, Mn and Al.
2) The film-forming temperature and free acid are important parameters for the compactness and corrosion resistance of the phosphate film. With increasing film-forming temperature, the density and the corrosion resistance are improved, but it will make plenty of residual in the phosphate solution. The decreasing of residual phosphide can be effectively controlled by adding free acid, and the corrosion resistance of the phosphate film has not been affected by this controlling.
3) The existence of the phosphate film has a great inhibitive action on anodic dissolution and restraint action on cathodic hydrogen evolution, which effectively improves the corrosion resistance of AZ31 magnesium alloy.
References
[1] KAINER K U, BALA SRINIVASAN P, BLAWERT C, DIETZEL W. 3.09-Corrosion of magnesium and its alloys [M]. Shreir’s Corrosion, 2010, 3: 2011-2041.
[2] SONG Guang-ling, XU Zhen-qing. The surface, microstructure and corrosion of magnesium alloy AZ31 sheet [J]. Electrochim Acta, 2010, 55: 4148-4161.
[3] CHENG Ying-liang, QUN Ting-wei, WANG Hui-ming, ZHANG Zhao. Comparison of corrosion behaviors of AZ31, AZ91, AM60 and ZK60 magnesium alloys [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 517-524.
[4] LIU Xiao-lan, ZHANG Tao, SHAO Ya-wei, MENG Guo-zhe, WANG Fu-hui. In-situ study of the formation process of stannate conversion coatings on AZ91D magnesium alloy using electrochemical noise [J]. Corros Sci, 2010, 52: 892-900.
[5] LI Qing, XU Shu-qiang, HU Jun-ying, ZHANG Shi-yan, ZHONG Xian-kang, YANG Xiao-kui. The effects to the structure and electrochemical behavior of zinc phosphate conversion coatings with ethanolamine on magnesium alloy AZ91D [J]. Electrochim Acta, 2010, 55: 887-894.
[6] BIERWAGEN G, BROWN R, BATTOCCHI D, HAYES S. Active metal-based corrosion protective coating systems for aircraft requiring no-chromate pretreatment [J]. Prog Org Coat, 2010, 67: 195-208.
[7] CUI Xue-fang, LI Ying, LI Qing-fen, JIN Guo, DING Ming-hui, WANG Fu-hui. Influence of phytic acid concentration on performance of phytic acid conversion coatings on the AZ91D magnesium alloy [J]. Mater Chem Phys, 2008, 111: 503-507.
[8] JIANG Yong-feng, ZHOU Hai-tao, ZENG Su-min. Microstructure and properties of oxalate conversion coating on AZ91D magnesium alloy [J]. Transaction of Nonferrous Metals Society of China, 2009, 19: 1416-1422.
[9] WANG C, ZHU S L, JIANG F, WANG F H. Cerium conversion coatings for AZ91D magnesium alloy in ethanol solution and its corrosion resistance [J]. Corros Sci, 2009, 51: 2916-2923.
[10] BARRANCO V, CARMONA N, GALVA’N J C, GROBELNY M, KWIATKOWSKI L, VILLEGAS M A. Electrochemical study of tailored sol-gel thin films as pre-treatment prior to organic coating for AZ91 magnesium alloy [J]. Prog Org Coat, 2010, 68: 347-355.
[11] NIU Li-yuan, LIN Ji-xing, SHI Zi-mu, XU Lin-chao. Improvement of anticorrosion and adhesion to magnesium alloy by phosphate coating formed at room temperature [J]. Transaction of Nonferrous Metals Society of China, 2010, 20: 1356-1360.
[12] ISHIZAKI T, SHIGEMATSU I, SAITO N. Anticorrosive magnesium phosphate coating on Magnesium alloy AZ31 [J]. Surf Coat Tech, 2009, 203: 2288-2291.
[13] SONG Ying-wei, SHAN Da-yong, CHEN Rong-shi, ZHANG Fan, HAN En-hou. Formation mechanism of phosphate conversion film on Mg-8.8Li alloy [J]. Corros Sci, 2009, 51: 62-69.
[14] CUI Xue-jun, WANG Xiu-chun, LU Jun-feng. A chromium-free phosphate solution and process for magnesium alloy: China Patent, ZL 2010 1 0123677.6 [P]. 2010-10-06. (in Chinese)
[15] CUI Xue-jun, ZHOU Ji-xue, LIN Xiu-zhou, LUO Hong, GONG Min. Growing process and formation mechanism of manganese phosphate conversion film of magnesium alloy AZ31 [J]. The Chinese Journal of Nanferrous Metals, 2012, 22(1): 15-21. (in Chinese)
[16] CHENG Ying-liang, WU Hai-lan, CHEN Zen-hua, WANG Hui-min, LI Ling-ling. Phosphating process of magnesium alloy AZ31 and corrosion resistance of coatings [J]. Transactions of Nonferrous Metals Society of China, 2006, 16: 1086-1091.
[17] ZANDI-ZAND R, ERSHAD-LANGROUDI A, RAHIMI A. Organic-inorganic hybrid coatings for corrosion protection of 1050 aluminum alloy [J]. J Noncryst, 2005, 351: 1307-1311.
[18] ZHONG Xian-kang, LI Qing, HU Jun-ying, LU Yi-hui. Characterization and corrosion studies of ceria thin film based on fluorinated AZ91D magnesium alloy [J]. Corros Sci, 2008, 50: 2304-2309.
崔学军1, 2,刘春海1, 2,杨瑞嵩1, 2,李明田1, 2,林修洲1, 2,龚 敏1, 2
1. 四川理工学院 材料腐蚀与防护四川省重点实验室,自贡 643000;
2. 四川理工学院 材料与化学工程学院,自贡 643000
摘 要:以磷酸二氢锰和无氟、无铬、无亚硝酸盐的添加剂为主要成分,通过化学沉积的方法在镁合金AZ31表面获得致密均匀的耐蚀磷化膜。通过硫酸铜点蚀测试、SEM、XRD及电化学极化曲线等表征手段,详细研究了膜层的形貌、组成、相结构及耐蚀性能,讨论了成膜温度和游离酸对膜层微结构、形貌及耐蚀性能的影响。结果表明,磷化膜通过抑制阳极溶解和阴极析氢,有效地提高了镁合金AZ31的耐蚀性能。
关键词:镁合金;AZ31合金;磷酸盐转化膜;无铬;耐蚀;阳极溶解;阴极析氢
(Edited by YANG Hua)
Foundation item: Projects (2011CL08, 2011CL01) supported by Open Fund of Material Corrosion and Protection Key Laboratory of Sichuan Province, China; Project (2011RC02) supported by Talent Introduction Funds of Sichuan University of Science and Engineering; Project (12ZA261) supported by Key Project of Education Department of Sichuan Province, China
Corresponding author: CUI Xue-jun; Tel: +86-813-5505860; E-mail: cxj_2046@163.com
DOI: 10.1016/S1003-6326(11)61522-7

