
Formation behavior of anodic TiO2 nanotubes in
fluoride containing electrolytes
Byung-Gwan LEE1, Jin-Wook CHOI1, Seong-Eun LEE1, Yong-Soo JEONG2, Han-Jun OH3, Choong-Soo CHI1
1. School of Advanced Materials Engineering, Kookmin University, Seoul, 136-702, Korea;
2. Korea Institute of Materials Science, Changwon, 641-831, Korea;
3. Department of Materials Science, Hanseo University, Seosan, 356-706, Korea
Received 18 June 2008; accepted 10 March 2009
Abstract: TiO2 nanotube layers can be formed with titanium in the electrolytes containing fluoride by electrochemical method. The role of fluoride ion, the crystallinity of the anodic oxide, and the chemical state were investigated. The results show the anodic film is composed of oxide and a little amount of hydroxide. The presence of F- ions leads to chemical dissolution of Ti oxide layer and prevents hydroxide precipitation. Consequently, chemical dissolution rate increases with increasing the fluoride content in the range of 0-2% (in mass fraction) because F- ions in electrolyte attack the interface and allow the ions of the electrolyte to easily penetrate into the interface. The as-anodized TiO2 nanotubes exhibit an amorphous structure. Thermally treated nanotubes are composed of mixtures of the anatase and rutile phases.
Key words: TiO2; nanotube; anodization; fluoride
1 Introduction
Self-organized nano-scale structures on metals or semiconductors have offered much attention due to the potential applications and the scientific interests. In the past few years, a novel form of valve metal oxide nanostructure has been developed based on anodization. Under specific anodic conditions in electrolytes containing fluoride, highly ordered arrays of oxide nanotubes can grow on various metals such as Ti[1], Zr[2], and W[3]. Particularly, TiO2 nanotubes formed on Ti have attracted great interests in recent years because of their variety of functional properties and potential applications, such as gas sensing[4], catalysis[5], solar cell[6], and biocompatible materials[7]. As an effective formation method of TiO2 nanotubes, the anodization process is useful because of low cost and simple fabrication.
In earlier research work, most of TiO2 nanotubes have been manufactured by anodization in aqueous HF-based electrolytes, but they grew only up to a length of about 500 nm. This limited growth may be assumed to be due to the fast dissolution process of the TiO2 nanotube layer rather than the formation of an oxide layer by the chemical oxidation process. Recently, the formation of TiO2 nanotubes in different electrolytes has been reported: aqueous organic solvent, acidic and neutral solution, all of which contain fluoride ions[8]. But, few detailed study has been published for the effect of fluoride in organic electrolytes on the growth of the nanotube. Thus, we studied the formation behavior of ordered TiO2 nanotubes anodized in glycerin electrolyte containing ammonium fluoride, and the effect of fluoride ion on the nanotube formation and phases of titania with useful physical and chemical properties.
2 Experimental
Titanium sheet (99.5% purity) with a size of 3 cm× 4 cm was cleaned by sonication in aceton, ethanol, and rinsed with deionized water for 10 min. The anodization to obtain TiO2 nanotubes was carried out in electrochemical cell by using a direct current source at a constant voltage of 20 V. The samples were anodized in mixture of glycerin+water (70?30 of volume ratio) with NH4F in the content range of 0-2% (in mass fraction). All electrolytes were prepared with reagent grade chemicals. Titania nanotube array was characterized by FE-SEM, XPS, XRD, etc.
3 Results and discussion
Fig.1(a) shows a top-view image of TiO2 nanotubes formed at 20 V for 1 h in NH4F mixture of glycerin and water (70?30 of volume ratio). Clearly, the anodization results in ordered porous structures with pore opening of approximately 100 nm in diameter. In order to measure the nanotube walls and ripples, TiO2 nanotubes were separated from the Ti substrate. As shown in Fig.1(b), the nanotubes were connected with each other, and the ripples occurred at sidewall of them after anodization. The origin of these ripples was considered the phenomenon that periodically repeated differences in the dissolution and oxide formation rate cause to leave some parts of the walls thicker or thinner than the other, thus leading to sidewall rippples[9]. The length and average outer diameter of nanotubes were 1 200 nm and 100 nm, respectively, and their aspect ratio was about 12. From Figs.1(a) and (c), it is apparent that the top ends of the tubes are open and the bottom ends of the tubes are closed. The closed bottoms of nanotube indicate the formation of a barrier layer structure similar to that of porous alumina. On the other hand, after anodization in fluoride-free electrolytes no porous structure is observed (Fig.1(d)).
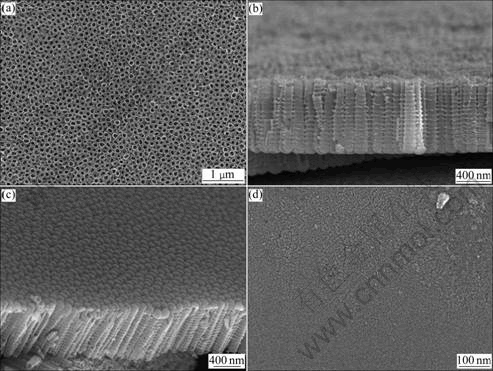
Fig.1 FE-SEM images of TiO formed at 20 V for 1 h: (a) Top-view; (b) Cross-sectional; (c) Bottom-view in glycerin+water (70?30 of volume ratio)+1.5% NH4F; (d) Top-view in glycerin+water (70:30 of volume ratio) without NH4F
Fig.2 shows current—time curves of Ti recorded during anodization in glycerin+water (70?30 of volume ratio) electrolyte with addition of different amounts of ammonium fluoride at 20 V. In fluoride-containing electrolytes obvious deviations from the curve in fluoride-free electrolyte are observed. The current densities in fluoride-containing electrolytes are higher than those in fluoride-free electrolyte, and increase with increasing the fluoride content in the range of 0-2%. This indicates that the dissolution reaction of the anodic oxide later is induced by F- ions. The growth of TiO2 nanotubes is a result of competition between electrochemical oxide formation and chemical dissolution of oxide by fluoride ions[8]. As can be seen from Fig.2, the formation of TiO2 nanotube is clearly affected by fluoride in the electrolyte. And a key factor that influences the electrochemistry is the fluoride concentration in the electrolyte. This can be ascribed to additional chemical dissolution of the electrochemically formed oxide layers due to soluble [TiF6]2- complexes. In the first step, the current density sharply decreases due to oxide formed on electrolyte/titanium interface with a low conductivity. The current densities start to increase by the actively chemical dissolution reaction due to F- ions from electrolyte. The porous structure is formed as a result of the localized chemical dissolution of the oxide by [TiF6]2- according to the reaction (1):
TiO2+F-+H+→[TiF6]2-+2H2O (1)
Ti+H2O→TiO2+H++e (2)
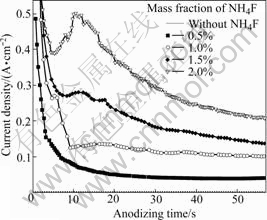
Fig.2 Current densities at 20 V during anodization in glycerin+ water (70?30 of volume ratio) solution with different amounts of NH4F
This leads to a higher field at bottom of the pore that drives further oxidation, and field assists dissolution where Ti ions come out of the metal and dissolve in solution[10]. Finally, the current reaches a steady state. This steady state current increases with increasing fluoride concentration[11].
However, if there is not F- ion in electrolyte, the oxide layer is composed of only compact layer instead of nanotubular layer according to the reaction (2). Because acidification from [TiF6]2- is not established at the electrolyte/oxide interface during the anodization, the presence of F- ions leads to chemical dissolution of TiO2 layer and prevents hydroxide precipitation of Ti4+ ions arriving at the electrolyte/oxide interface, so Ti4+ can be complexed to [TiF6]2- before reacting with a hydroxide layer. From the results of XPS measurement, the O 1s spectra (Fig.3) about the chemical state of the TiO2 nanotubes clearly show that the anodic film consists of oxide (530.1 eV) and a little amount of hydroxide (531.9 eV).
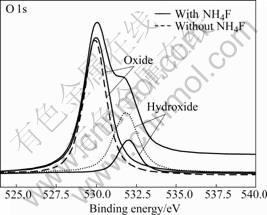
Fig.3 XPS spectra of TiO2 nanotubes formed at 20 V for 1 h in glycerin+water (70?30 of volume ratio)+1.5% NH4F
The phases of TiO2 nanotubes include anatase, rutile and brookite. The structural features of these phases can be explained on the basis of different types of linkages of Ti—O octahedral units[12]. The as-anodized TiO2 nanotubes are in amorphous structure. Meanwhile, annealed TiO2 nanotubes are converted to anatase at approximately 280 ℃ or mixture of anatase and rutile at temperatures higher than 450 ℃[13]. From Fig.4(a) it can be seen that, the XRD results of as-anodized titania films show only titanium peaks at 2θ=52.95? (102), 70.4? (103), and 76.05? (112) that are originated from the Ti substrate. After annealing at 450 ℃ (Fig.4(b)) for 60 min, anatase phase of TiO2 nanotubes appears. After annealing at 550 ℃ (Fig.4(c)), the peaks related to rutile appear, but most of these phases are still composed of anatase. And then rutile phase is dominantly observed more at 650 ℃ (Fig.4(d)) than that at 550 ℃. With the increasing of annealing temperature, rutile peaks become more numerous and strong. The result shows that the various crystal transition of the anatase-rutile phases from amorphous takes place after additional annealing treatment.
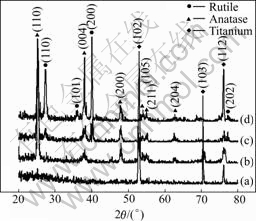
Fig.4 X-ray diffraction patterns of TiO2 nanotubes after heat treatment at different temperatures for 1 h: (a) As-anodized; (b) 450 ℃; (c) 550 ℃; (d) 650 ℃
4 Conclusions
1) Self-ordered TiO2 nanotubes can be formed by anodization for 1 h in glycerin electrolyte with addition of NH4F. In fluoride-containing electrolyte, the current densities are higher than those of fluoride-free electrolyte, and increase with increasing the fluoride content in the range of 0-2%. Only compact layer is formed in fluoride-free electrolyte.
2) In fluoride-containing electrolyte, nanotubular layer is formed on Ti substrate. The additional chemical dissolution of the electrochemically formed oxide layers is due to soluble [TiF6]2- complexes. Thereby, to achieve self-ordered TiO2 nanotube, the presence of fluoride in electrolyte is required.
3) The present work shows the chemical composition between phases of the TiO2 nanotubes. These indicate that the anodic film is composed of oxide and hydroxide, and the transition of the anatase-rutile phases from amorphous takes place after additional annealing. Clearly, heat treatment has a significant influence on phase transition of TiO2 nanotubes.
References
[1] GHICOV A, TSUCHIYA H, MACAK J M, SCHMUKI P. Titanium oxide nanotubes prepared in phosphate electrolytes [J]. Electrochemistry Communications, 2005, 7: 505-509.
[2] Tsuchiya h, Schmuki p. Thick self-organized porous zirconium oxide formed in H2SO4/NH4F electrolytes [J]. Electrochemistry Communications, 2004, 6: 1131-1134.
[3] Tsuchiya h, Macak j m, Sieber i, Taveira l, Ghicov a, Sirotna k, Schmuki p. Self-organized porous WO3 formed in NaF electrolytes [J]. Electrochemistry Communications, 2005, 7: 295-298
[4] Paulose m, Varghese O K, Mor g k, Grimes c a, Ong k g. Unprecedented ultra-high hydrogen gas sensitivity in undoped titania nanotubes [J]. Nanotechnology, 2006, 17: 398-402.
[5] Macak J M, Schmidt-Stein f, Schmuki f. Efficient oxygen reduction on layers of ordered TiO2 nanotubes loaded with Au nanoparticles [J]. Electrochemistry Communications, 2007, 9: 1783-1787.
[6] Onodaa k, Ngamsinlapasathiana s, Fujiedaa t, Yoshikawa s. The superiority of Ti plate as the substrate of dye-sensitized solar cells [J]. Solar Energy Materials and Solar Cells, 2007, 91: 1176-1181.
[7] OH H J, LEE J H, JEONG Y, KIM Y J, CHI C S. Microstructural characterization of biomedical titanium oxide film by electrochemical method [J]. Surface and Coating Technology, 2005, 198: 247-252.
[8] Yasuda k, Macak j m, Berger s, Ghicov a, Schmuki p. Mechanistic aspects of the self-organization process for oxide nanotube formation on valve metals [J]. Journal of Electrochemical Society, 2007, 154: C472-C478.
[9] Macak J M, Hildebrand H, Marten-Jahns U, Schmuki P. Mechanistic aspects and growth of large diameter self-organized TiO2 nanotubes [J]. Journal of Electroanalytical Chemistry, 2008, 621: 254-266.
[10] Cai q, Paulose m, Varghese o k, Grimes c a. The effect of electrolyte composition on the fabrication of self-organized titanium oxide nanotube arrays by anodic oxidation [J]. Journal of Materials Research Society, 2005, 20: 230-236.
[11] Beranek r, Hildebrand h, Schmuki p. Self-organized porous titanium oxide prepared in H2SO4/HF electrolytes[J]. Electrochemical and Solid-State Letters, 2003, 6: B12-B14.
[12] Godbole v p, Kim Y s, Kim g s, Dar m a, Shin h s. Synthesis of titanate nanotubes and its processing by different methods [J]. Electrochemical Acta, 2006, 52: 1781-1787.
[13] Ghicov a, Tsuchiya h, Macak j m, Schmuki p. Annealing effects on the photoresponse of TiO2 nanotubes [J]. Physica Status Solidi, 2006, 203: R28-R30.
Corresponding author: Choong-Soo CHI; Tel: +82-2-910-4666; E-mail: cschi@kookmin.ac.kr
DOI: 10.1016/S1003-6326(08)60361-1
(Edited by YANG Hua)