铜冶炼烟尘的控电位氧化浸出
来源期刊:中国有色金属学报(英文版)2018年第9期
论文作者:刘伟锋 傅新欣 杨天足 张杜超 陈霖
文章页码:1854 - 1861
关键词:铜;冶炼烟尘;氧化浸出;电位
Key words:copper; smelting dust; oxidation leaching; potential
摘 要:采用氧化浸出和电位控制技术从铜冶炼烟尘中浸出金属,研究H2O2用量、H2O2加入速度、初始盐酸浓度、浸出温度、初始液固比和浸出时间对金属浸出率的影响。最终得到最优浸出条件为:H2O2用量0.8 mL/g(氧化还原电位为429 mV)、H2O2加入速度1.0 mL/min、初始硫酸浓度1.0 mol/L、初始盐酸浓度1.0 mol/L、浸出温度80 °C、初始液固比5:1 mL/g以及浸出时间1.5 h。在此最优条件下,铜冶炼烟尘中的铜和砷能被有效地浸出,剩下的浸出渣可作为一种合适的铅冶炼资源。此时,铜、砷和铁的平均浸出率分别为95.27%、96.82%和46.65%。
Abstract: A study was conducted for metal extraction from copper smelting dust using the oxidation leaching and control of potential technology. The effects of H2O2 dosage, H2O2 feeding speed, initial HCl concentration, leaching temperature, liquid-to-solid ratio and leaching time on metals leaching efficiencies were investigated. The following optimized leaching conditions were obtained: H2O2 dosage of 0.8 mL/g (redox potential of 429 mV), H2O2 feeding speed of 1.0 mL/min, initial H2SO4 concentration of 1.0 mol/L, initial HCl concentration of 1.0 mol/L, leaching temperature of 80 °C, initial liquid-to-solid ratio of 5:1 mL/g and leaching time of 1.5 h. Under the optimized conditions, copper and arsenic can be effectively leached from copper smelting dust, leaving residue as a suitable lead resource. The average leaching efficiencies of copper, arsenic and iron are 95.27%, 96.82% and 46.65%, respectively.
Trans. Nonferrous Met. Soc. China 28(2018) 1854-1861
Wei-feng LIU, Xin-xin FU, Tian-zu YANG, Du-chao ZHANG, Lin CHEN
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 16 May 2017; accepted 8 September 2017
Abstract: A study was conducted for metal extraction from copper smelting dust using the oxidation leaching and control of potential technology. The effects of H2O2 dosage, H2O2 feeding speed, initial HCl concentration, leaching temperature, liquid-to-solid ratio and leaching time on metals leaching efficiencies were investigated. The following optimized leaching conditions were obtained: H2O2 dosage of 0.8 mL/g (redox potential of 429 mV), H2O2 feeding speed of 1.0 mL/min, initial H2SO4 concentration of 1.0 mol/L, initial HCl concentration of 1.0 mol/L, leaching temperature of 80 °C, initial liquid-to-solid ratio of 5:1 mL/g and leaching time of 1.5 h. Under the optimized conditions, copper and arsenic can be effectively leached from copper smelting dust, leaving residue as a suitable lead resource. The average leaching efficiencies of copper, arsenic and iron are 95.27%, 96.82% and 46.65%, respectively.
Key words: copper; smelting dust; oxidation leaching; potential
1 Introduction
Copper smelting dust is generated in the pyro- metallurgical process of copper extraction, which contains both several valuable metals such as copper, lead, zinc and bismuth and undesirable toxic materials like arsenic and cadmium [1,2]. In recent years, the increase of copper production and depletion of high grade copper ores have resulted in a higher amount of dust. Due to its high metal content and environmental impact, copper smelting dust is regarded as a secondary resource, which should be comprehensively utilized to achieve the maximum economic benefits [3]. If copper smelting dust is directly sent back to smelting process to recover metals, the quality of electric copper and the processing capability of furnace will be reduced greatly owning to the circulating accumulation of the impurities [4]. Therefore, it is necessary to treat smelting dust individually to recover the metals.
The conventional treatment of smelting dust can be mainly classified into three methods: pyro-metallurgical, hydrometallurgical and hybrid process. With pyro-metallurgical process, dust is smelted in a side-blowing furnace or blast furnace, in which lead, bismuth, gold and silver can be enriched in crude lead and arsenic volatilizes into the flue dust [5]. This process can reduce the impact of dust on the main smelting process, but there are some problems, such as the dispersal of metals, high energy consumption and environmental pollution. Therefore, extensive studies have been carried out on the treatment of smelting dust by hydrometallurgical processes [6]. Many leaching agents are applied in the dust leaching, including water, sulfuric acid, zinc electrolyte, sodium hydroxide and ammonia, and the leaching efficiencies can be increased further by the addition of H2O2, pressure leaching and Cl2/Cl- systems [7-9]. XU et al [10] reported that copper and zinc can be effectively separated from arsenic and iron in the pressure leaching, while few work was performed to recover arsenic, lead and bismuth. For most of hydrometallurgical processes, it is very difficult to realize industrial production and practical application due to their shortcomings, such as long flow, complex process and low efficiency. At present, some plants in China treat copper smelting dust by a unite technique of hydro- and pyro-metallurgy, where dust is firstly leached by water, sulfuric acid or sodium sulfide and then the leaching residues are sent to lead smelting process. Moreover, some researches focused on the removal of arsenic by anoxic roasting or reducing roasting and then the roasted product is leached in the hydrometallurgical process [11]. However, lead, arsenic and zinc will be volatilized as mixture dust, and the collected As2O3 dust may result in a source of secondary pollution.
In recent years, bottom-blowing furnace matte smelting technology has been developed greatly in China. Due to the special structure of the bottom- blowing furnace, the copper smelting dust has the characteristics of complex components and high-arsenic, which undoubtedly makes it more difficult to be treated [12,13]. Therefore, the process of oxidation leaching by controlling potential was proposed to treat bottom-blown furnace smelting dust in this work. And the factors affecting the leaching efficiencies of copper, arsenic and iron, such as H2O2 dosage, H2O2 feeding speed, initial HCl concentration, leaching temperature, liquid-to-solid ratio and leaching time were optimized.
2 Experimental
2.1 Materials and reagents
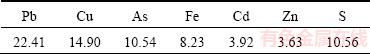
The source of the copper smelting dust used in this study was from a copper smelter in Henan Province, China. And the samples were obtained in the bottom- blowing furnace matte smelting process. The samples were dried, ground and sieved, which ensured all of them passing through 200-mesh sieve. Analytical determination of the dust contents is shown in Table 1. The results demonstrate that the major elements present in the dust are lead (22.41%), copper (14.90%), arsenic (10.54%) and iron (8.23%).
Table 1 Main chemical composition of copper smelting dust (mass fraction, %)
As presented in Fig. 1, the XRD results suggest that lead sulfate (PbSO4) is the main mineral phase, and arsenic trioxide (As2O3), iron oxide (Fe2O3), magnetite (Fe3O4), copper sulfate (CuSO4), copper sulfide (CuS) and copper oxide (CuO) are identified as the dust components. The chemical phase analysis of metals is listed in Table 2 [14,15]. The results show that phase compositions of copper are very complex, mainly including CuSO4, CuO and CuS [16]. A large quantity of lead exists in the form of PbSO4. However, arsenic and iron in the dust mainly exist in the form of oxides, which reach 83.76% and 77.27%, respectively. The SEM-EDS images of the dust are shown in Fig. 2. It can be seen that the distribution of composition and particle size are uniform. The results of EDS indicate that metal contents in the dust are consistent with the results listed in Table 1.
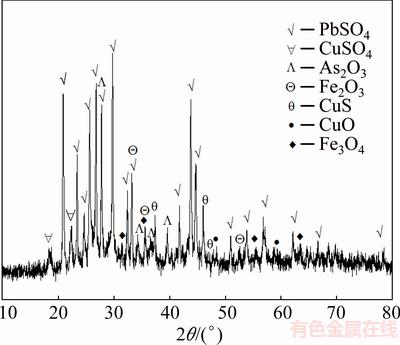
Fig. 1 XRD pattern of copper smelting dust
Table 2 Chemical phase analysis of metals in copper smelting dust (mass fraction, %)
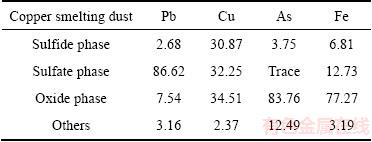
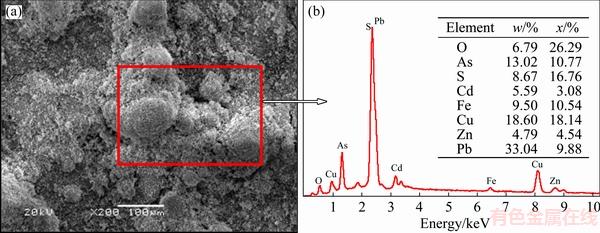
Fig. 2 SEM image (a) and corresponding EDS pattern (b) of copper smelting dust
All the used reagents that sulfuric acid (98%), hydrochloric acid (36%) and hydrogen peroxide (30%) are of analytical grade.
2.2 Experimental procedure
The leaching experiments were conducted in a 500 mL flask equipped with a mechanical stirrer, a mercury thermometer and a potentiometer (Fig. 3). And the flask was placed in a thermostatic water bath and the temperature was controlled accurately within ±0.5 °C. For each experiment, 50 g dust was mixed with leaching reagent containing desired concentrations of H2SO4 and HCl when the temperature reached the pre-set value. The desired amount of H2O2 was pumped into the slurry by a constant-flow pump, and then the slurry was leached at a stirring speed of 400 r/min. The redox potential of the solution was monitored by a MT320-SpH instrument with a platinum electrode as the working electrode and a calomel electrode as the reference electrode. After the reaction, the slurry was filtered, and the residue was washed by hot water. The washed residue was weighed after being dried at 110 °C for 24 h and then subjected to analysis. And the resulting leaching solution mixed with the washing water was used for the recovery of copper, arsenic and iron. The extraction rates of metals were calculated on the basis of residue composition.
In order to determine the suitable leaching system, the dust was subjected to treatment with different leaching reagents, such as water, sulfuric acid, mixed acid (sulfuric acid and hydrochloric acid), sulfuric acid and hydrogen peroxide, hydrochloric acid and hydrogen peroxide, and mixed acid and hydrogen peroxide. These different treatments were carried out under the following consistent conditions: leaching temperature of 80 °C, liquid-to-solid ratio of 4:1 mL/g and leaching time of 1.5 h. Secondly, the effects of H2O2 dosage, H2O2 feeding speed, initial HCl concentration, leaching temperature, liquid-to-solid ratio and leaching time on metal leaching efficiencies were investigated.
2.3 Analysis methods
The contents of metals in dust and leaching residue were determined by ICP-MS (IRIS Intrepid II XSP, US). The phases of dust and leaching residue were analyzed by X-ray diffraction (TTRAX-3, 50 kV, 300 mA, 10 (°)/min, Japan). Morphology of samples and energy spectra were characterized by SEM (JSM-6360LV, 25 kV, Japan) and EDS (EDX-GENESIS, 60S, USA), respectively. Sulfur was measured with a sulfur and carbon analyzer (LECOSC-444, US).
3 Results and discussion
3.1 Effect of leaching system
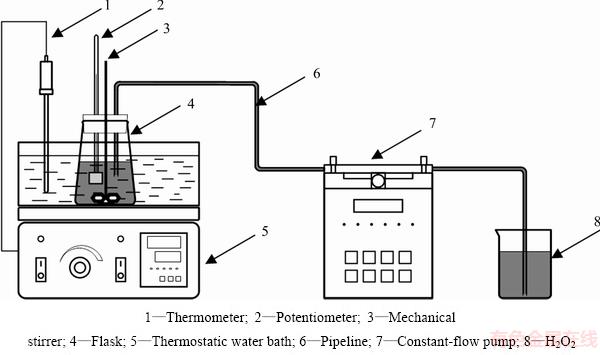
Fig. 3 Experimental set-up for oxidation leaching of copper smelting dust
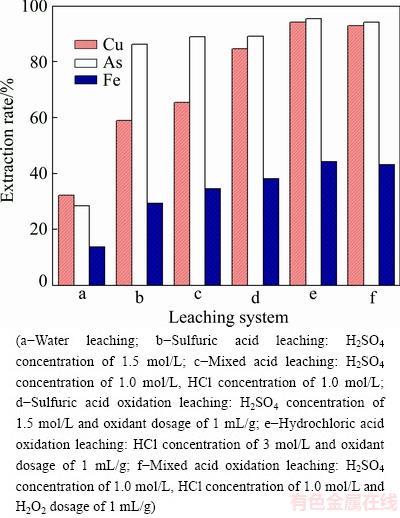
Fig. 4 Effect of leaching system on metal extraction rate
Figure 4 illustrates that the extraction rates of metals with water as leaching agent are low. When the dust is leached in sulfuric acid, the extraction rates of copper, arsenic and iron are 59.03%, 86.29%, and 29.46%, respectively. This can be attributed to that CuSO4, CuO, As2O3 and Fe2O3 can be directly dissolved in sulfuric acid. Appropriate HCl concentration can further improve the extraction rates of copper, arsenic and iron. And the filtration rate will speed up with the addition of HCl (data not shown). However, high concentration of HCl can lead to dissolution of lead and corrosion of equipment. Additionally, oxidants are required for the dissolution of metal sulfides. And it is necessary to convert trivalent arsenic to pentavalent arsenic, which is conducive to the subsequent separation of copper and arsenic in the leaching solution with sulfide precipitation method. Hydrogen peroxide is a good oxidizing agent and can be used for dust leaching because its oxidation potential (1.77 V) is adequate for oxidizing almost all the metal sulfide. The oxidative action of hydrogen peroxide in acidic solution is based on its reduction [17]. Therefore, oxidation leaching with mixed acid as leaching agent was selected for the treatment of copper smelting dust. In this leaching system, approximately 92.83% of Cu, 94.17% of As and 44.32% of Fe in the dust are leached. The probable main reactions in the leaching with H2O2 as the oxidant are as follows [18]:
CuO+2H+→Cu2++H2O (1)
Fe2O3+6H+→2Fe3++3H2O (2)
H2O2+2H++2e→2H2O (3)
CuS+H2O2+2H+→Cu2++S+2H2O (4)
As2O3+H2O+2H2O2→2H3AsO4 (5)
3.2 Effect of H2O2 dosage
H2O2 dosage is the most important factor in metal extraction. Figure 5 shows the effect of H2O2 dosage (where mL/g represents the ratio of added volume of 30% H2O2 to the mass of dust) on the extraction rates of metals in the leaching process.
It can be seen from Fig. 5 that the extraction rate of copper is raised from 65.46% to 92.75% with the increase of H2O2 dosage from 0 to 0.8 mL/g. Further increasing H2O2 dosage, the extraction rate of copper remains relatively steady. The extraction rate of arsenic does not change obviously with the increase of H2O2 dosage. The extraction rate of iron increases with the increase of H2O2 dosage from 0 to 0.4 mL/g and raises slightly with further increasing H2O2 dosage. This can be attributed to that it is beneficial to the dissolution of sulfide species by adding H2O2, whereas magnetite is not easily dissolved in the leaching solution [19,20]. Therefore, the H2O2 dosage of 0.8 mL/g is required to achieve satisfactory leaching results.
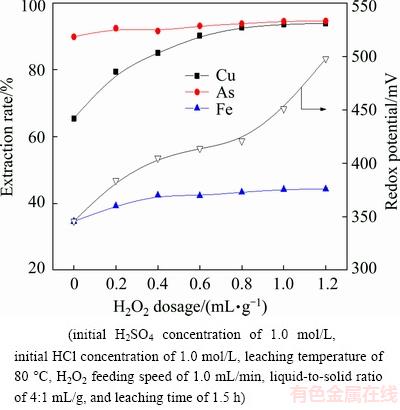
Fig. 5 Effects of H2O2 dosage on metal extraction rate and redox potential
In addition, the redox potential of the leaching solution gradually increases by improving the H2O2 dosage. It should be noted that the redox potential increases rapidly from 346 to 405 mV when the H2O2 dosage increases from 0 to 0.4 mL/g, which corresponds to the increase of metal extraction rates. When the H2O2 dosage is between 0.4 and 0.8 mL/g, the redox potential has a slight increase. However, the redox potential improves significantly from 421 to 498 mV when the H2O2 dosage increases from 0.8 to 1.2 mL/g. This is largely attributable to the excessive H2O2. Based on the above results, the metal extraction rates can be judged effectively by observing the redox potential. And the redox potential is determined by the appropriate H2O2 dosage.
3.3 Effect of H2O2 feeding speed
The effect of H2O2 feeding speed on the extraction rates of metals is presented in Fig. 6. It can be seen that, the extraction rates of arsenic and iron change insignificantly with the increase of H2O2 feeding speed. However, the H2O2 feeding speed has a significant effect on the copper dissolution. The extraction rate of copper is raised from 88.13% to 92.75% with the increase of H2O2 feeding speed from 0.5 to 1.0 mL/min. This may be due to the fact that the added H2O2 cannot fully react with dust in a short time. The extraction rate of copper decreases slowly with further increasing H2O2 feeding speed. It is mainly caused by the fact that the decomposition rate of H2O2 increases with the increase of H2O2 feeding speed. The change of redox potential of the solution also validates the phenomenon, which decreases from 461 to 364 mV with accelerating the addition of H2O2. Therefore, the appropriate feeding speed of H2O2 is chosen to be 1.0 mL/min.
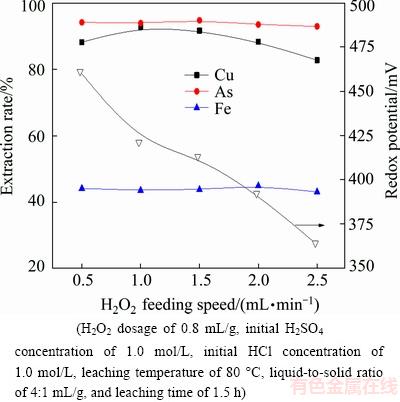
Fig. 6 Effect of H2O2 feeding speed on metal extraction rate and redox potential
3.4 Effect of initial HCl concentration
Figure 7 presents the effect of initial HCl concentration on metal extraction. The leaching efficiencies of copper, arsenic and iron increase apparently with the increase of initial HCl concentration and reach 92.75%, 93.85% and 43.39%, respectively, when the initial HCl concentration is up to 1.0 mol/L, and remain relatively stable with further increasing. The redox potential initially increases with increasing initial HCl concentration, and then remains relatively steady. The probable reasons are as follows: 1) Chlorine ions were used as ligands bind with metal ions to form stable complexes in the leaching solution, which is favorable for metal leaching [21] ; 2) Some studies showed that Cl- can enhance the activity of H+ in leaching solutions. Considering the extraction rates of metals, the appropriate HCl concentration is chosen to be 1.0 mol/L.
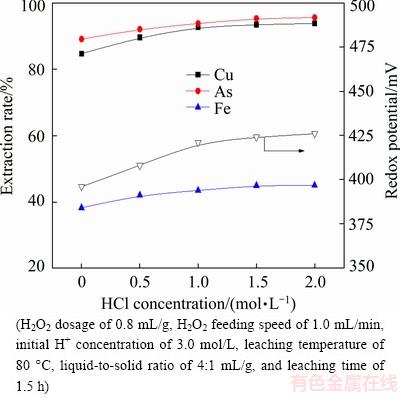
Fig. 7 Effect of initial HCl concentration on metal extraction
3.5 Effect of leaching temperature
The effect of leaching temperature on the extraction rates of metals is shown in Fig. 8. The results indicate that the leaching efficiencies of copper, arsenic and iron increase with leaching temperature. With the increase of leaching temperature from 50 to 90 °C, the extraction rates of copper, arsenic and iron increase from 78.91%, 87.27% and 23.41% to 93.64%, 95.01% and 45.64%, respectively. Correspondingly, the redox potential increases from 366 to 434 mV. This is due to the fact that the increase of temperature is beneficial to the diffusion of material. Additionally, the solubilities of copper, arsenic and iron increase with the increase of leaching temperature. On the other hand, H2O2 has poor reactivity under the low temperature and cannot effectively react with the dust [22]. Last but not least, some of the multivalent metal ions can be oxidized by hydrogen peroxide and react with metal sulfides in dust, which will allow hydrogen peroxide to be fully utilized at a higher temperature. Given consideration on metal extraction and energy consumption, 80 °C is determined to be the optimum leaching temperature.
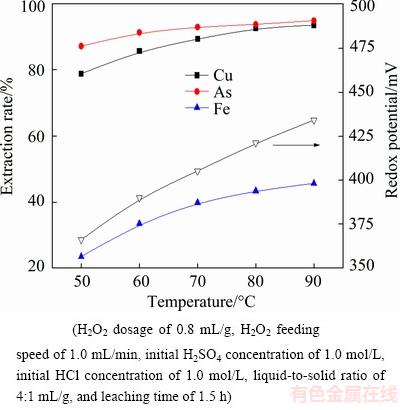
Fig. 8 Effect of leaching temperature on metal extraction rate and redox potential
3.6 Effect of liquid-to-solid ratio
The effect of liquid-to-solid ratio on the extraction rates of metals is shown in Fig. 9. The results reveal that liquid-to-solid ratio plays an important role in the metal leaching. With the increase of liquid-to-solid ratio from 3:1 to 5:1 mL/g, the extraction rates of copper, arsenic and iron increase from 82.37%, 85.73% and 32.92% to 95.64%, 97.12% and 46.81%, respectively. Further increasing has a marginal effect on metal extraction rates. The redox potential of the leaching solution initially increases with increasing liquid-to-solid ratio, but remains relatively steady for the liquid-to-solid ratio above 5:1 mL/g. This can be explained by that the increased volume of leaching reagent can promote the mass transfer process of the solid–liquid interface [23,24]. Considering the reagent consumption and the volume of leaching solution, the optimized liquid-to- solid ratio is chosen to be 5:1 mL/g.
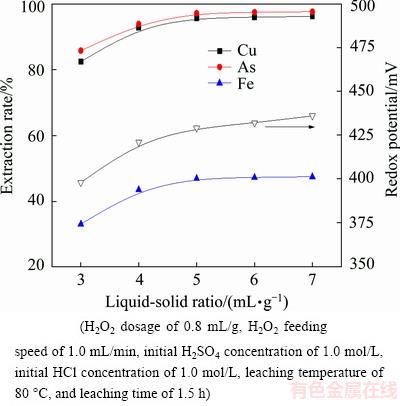
Fig. 9 Effect of liquid-to-solid ratio on metal extraction rate and redox potential
3.7 Effect of leaching time
Figure 10 shows the effect of leaching time on the extraction rates of metals and the redox potential. Results indicate that the leaching processes of metals are affected obviously by leaching time. With the leaching time increasing from 1 h to 1.5 h, the extraction rates of copper, arsenic and iron increase from 82.17%, 86.21% and 34.19% to 95.64%, 97.12% and 46.81%, respectively. Further prolonging leaching time, the extraction rates of metals have only negligible change. Given higher metal extraction rates and lower energy consumption, the leaching time is fixed at 1.5 h.
3.8 Oxidation leaching by controlling potential and residue characterization
Based on the above mentioned single-factor experiments, the optimum conditions are determined as: H2O2 dosage of 0.8 mL/g, H2O2 feeding speed of 1.0 mL/min, initial H2SO4 concentration of 1.0 mol/L, initial HCl concentration of 1.0 mol/L, leaching temperature of 80 °C, liquid-to-solid ratio of 5:1 mL/g and leaching time of 1.5 h. In addition, the oxidation leaching process can be improved by combination with control of the redox potential. Therefore, the process of oxidation leaching by controlling potential was proposed. In this process, the redox potential is determined by the appropriate H2O2 dosage. And the redox potential of 429 mV is required to achieve satisfactory leaching results.
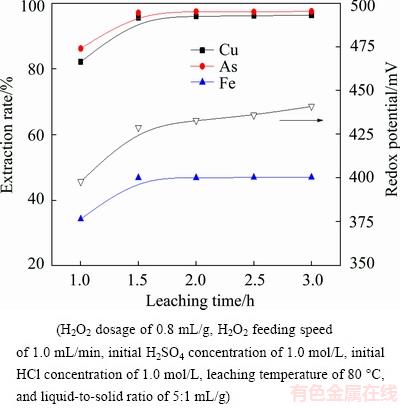
Fig. 10 Effect of leaching time on metal extraction rate and redox potential
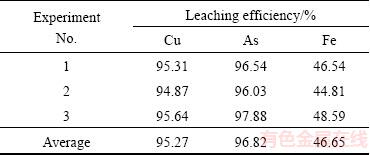
Under the optimum conditions mentioned above, three integrated experiments were conducted by controlling the redox potential, and the results are listed in Table 3. In these experiments, approximately 47.3% of residue was obtained. Additionally, the average leaching efficiencies of copper, arsenic and iron are 95.27%, 96.82% and 46.65%, respectively.
Table 3 Results of integrated experiments
The chemical composition of the leaching residue is listed in Table 4. The contents of copper, arsenic and iron in the leaching residue are 1.39%, 0.71% and 9.34%, respectively. The XRD and SEM-EDS analyses of the leaching residue are shown in Figs. 11 and 12, respectively. The XRD pattern of leach residue shows that the main phases in the leaching residue are PbSO4, Fe3O4 and SiO2, and no phase containing copper or arsenic is detected, which prove that the extraction of copper and arsenic from copper smelting dust is good. Heavy elements, whose atomic number is high, will appear brighter in the image than light elements, owing to their strong backscatter electrons. Figure 12 shows that some bright particles (Zone A) contain a high Pb content; and some dark particles (Zone B) are composed of iron, lead, silicon and oxygen, and they are a mixture of Fe3O4, PbSO4 and SiO2. Given consideration on a better economic value and lower arsenic content, the leaching residue can be returned to lead smelting process.
Table 4 Main chemical composition of leaching residue under optimum conditions (mass fraction, %)
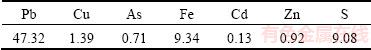
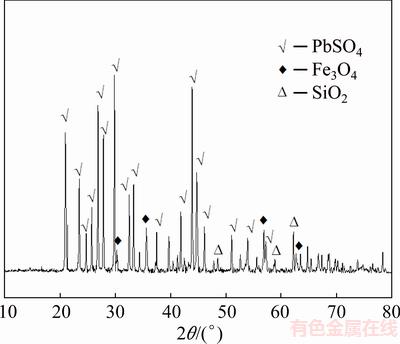
Fig. 11 XRD pattern of leaching residue
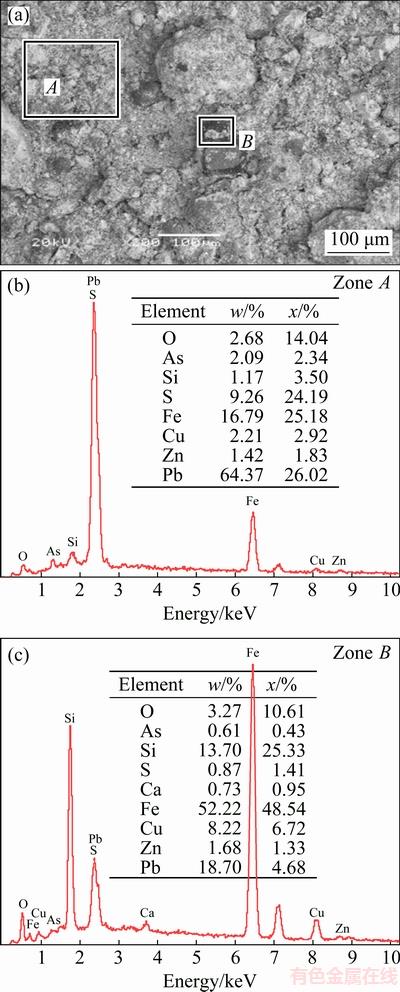
Fig. 12 SEM image (a) and corresponding EDS patterns (b, c) of leaching residue
4 Conclusions
1) According to the results of single-factor experiments, the optimal leaching conditions were obtained as follows: H2O2 dosage of 0.8 mL/g (redox potential of 429 mV), H2O2 feeding speed of 1.0 mL/min, initial H2SO4 concentration of 1.0 mol/L, initial HCl concentration of 1.0 mol/L, leaching temperature of 80 °C, liquid-to-solid ratio of 5:1 mL/g and leaching time of 1.5 h.
2) Under these optimum conditions, the average leaching efficiencies of copper, arsenic and iron are 95.27%, 96.82% and 46.65%, respectively. The main chemical compositions in the leaching residue are PbSO4, Fe3O4 and SiO2, and the leaching residue can be applied as raw material to a lead smelting plant.
3) The oxidation leaching process can be improved by combination with control of the redox potential. The appropriate amount of H2O2 can be determined by the redox potential of the leaching solution, and the end point of the reaction can be judged effectively.
References
[1] MONTENEGRO V, SANO H, FUJISAWA T. Recirculation of high arsenic content copper smelting dust to smelting and converting processes [J]. Minerals Engineering, 2013, 49(8): 184-189.
[2] MIN Xiao-bo, LIAO Ying-ping, CHAI Li-yuan, YANG Zhi-hui, XIONG Shan, LIU Lin, LI Qing-zhu. Removal and stabilization of arsenic from anode slime by forming crystal scorodite [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(4): 1298-1306.
[3] GUO Xue-yi, YI Yu, SHI Jing, TIAN Qing-hua. Leaching behavior of metals from high-arsenic dust by NaOH-Na2S alkaline leaching [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(2): 575-580.
[4] CHEN Ya, LIAO Ting, LI Gai-bian, CHEN Bai-zhen, SHI Xi-chang. Recovery of bismuth and arsenic from copper smelter flue dusts after copper and zinc extraction [J]. Minerals Engineering, 2012, 39(12): 23-28.
[5] GAO Da-yin, ZHOU Ming, XIA Zhao-quan, ZHANG Xin-hong, LIU Zhe, LIN Gong-mian. A two-stage process for treating copper dust containing lead in side-blowing furnace: CAP patent, CN103757423A [P]. 2014-01-27. (in Chinese)
[6] KUL M, OSKAY K O, SIMSIR M, SUBUTAY H, KIRGEZEN H. Optimization of selective leaching of Zn from electric arc furnace steelmaking dust using response surface methodology [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(8): 2753-2762.
[7] ALGUACI F J, GARCIA D I, LOPEZ F. Recycling of copper flue dust via leaching-solvent extraction processing [J]. Desalination and Water Treatment, 2015, 10(56): 1202-1207.
[8] LI Qiang, PINTO I S S, ZHAO You-cai. Sequential stepwise recovery of selected metals from flue dusts of secondary copper smelting [J]. Journal of Cleaner Production, 2014(84): 663-670.
[9] MORALES A, CRUELLS M, ROCA A, BERGO, R. Treatment of copper flash smelter flue dusts for copper and zinc extraction and arsenic stabilization [J]. Hydrometallurgy, 2010, 105(1-2): 148-154.
[10] XU Zhi-Feng, LI Qiang, NIE, Hua-Ping. Pressure leaching technique of smelter dust with high-copper and high-arsenic [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: s176-s181.
[11] SHIBAYAMA A, TAKASAKI Y, WILLIAM T, YAMATODANI A, HIGUCHI Y, SUNAGAWA S, ONO E. Treatment of smelting residue for arsenic removal and recovery of copper using Pyro-hydrometallurgical process [J]. Journal of Hazardous Materials, 2010, 181: 1016-1023.
[12] GUO Xue-yi, WANG Qin-meng, TIAN Qing-hua, ZHAO Bao-jun. Analysis and optimization of oxygen bottom blowing copper smelting process [J]. The Chinese Journal of Nonferrous Metals, 2016, 26(3): 689-698. (in Chinese)
[13] LIU Liu, YAN Hong-jie, ZHOU Jie-min, GAO Qiang, ZHANG Zhen-yang, LIU Fang-kan, CUI Zhi-xiang. Mechanism of copper smelting process by oxygen bottom blowing and microanalysis of smelting products [J]. The Chinese Journal of Nonferrous Metals, 2012(7): 2116-2124. (in Chinese)
[14] MA Miao, HUANG Yu-dai, GUO Yong, JIA Dian-zeng, TANG Xin-cun, WANG Xing-chao, PAN Yan-liang. Determination of speciation in white ash and acid leaching arsenic removal technique [J]. Chinese Journal of Applied Chemistry, 2015, 32(10): 1208-1214. (in Chinese)
[15] XIA Bing-wei. The study of lead phase analytical method and its application [J]. Hunan Nonferrous Metals, 2013, 29(05): 66-68. (in Chinese)
[16] TURAN M D. Direct selective leaching of chalcopyrite concentrate [J]. Canadian Metallurgical Quarterly, 2014, 53(4): 444-449.
[17] TURAN M D, ALTUNDOGAN H S. Leaching of chalcopyrite concentrate with hydrogen peroxide and sulfuric acid in an autoclave system [J]. Metallurgical and Materials Transactions B, 2013, 44(4): 809-819.
[18] MAHAJAN V, MISRA M, ZHONG K, FUERSTENAU, M C. Enhanced leaching of copper from chalcopyrite in hydrogen peroxide-glycol system [J]. Minerals Engineering, 2007, 20(7): 670-674.
[19] HUA Yi-xin. Introduction to nonferrous metallurgy [M]. 3nd ed. Beijing: Metallurgical Industry Press, 2014. (in Chinese)
[20] KIM Jae-Kyeong, OH Han-Sang, JO Chang-Wha, SUH Yong-Jae, JANG Hee-Dong, Koo Kee-Kahb. Recovery of iron as a form of ferrous acetate precipitates from low-grade magnetite ore [J]. Chemical Engineering Research and Design, 2010, 88(11): 1467-1473.
[21] TANG Mo-tang, YANG Tian-zu. Fundamental and technology of complex metallurgy [M]. 1nd ed. Changsha: Central South University Press, 2011. (in Chinese)
[22] LIU Wei-feng, RAO Shuai, WANG Wen-yao, YANG Tian-zu, YANG Lin, CHEN Lin, ZHANG Du-chao. Selective leaching of cobalt and iron from cobalt white alloy in sulfuric acid solution with catalyst [J]. International Journal of Mineral Processing, 2015, 141: 8-14.
[23] CHEN Tao, LEI Chang, YAN Bo, XIAO Xian-ming. Metal recovery from the copper sulfide tailing with leaching and fractional precipitation technology [J]. Hydrometallurgy, 2014, 147-148: 178-182.
[24] LI Hong-gui. Metallurgical principle [M]. 1nd ed. Beijing: China Sciences Publishing House,2005. (in Chinese)
刘伟锋,傅新欣,杨天足,张杜超,陈 霖
中南大学 冶金与环境学院,长沙 410083
摘 要:采用氧化浸出和电位控制技术从铜冶炼烟尘中浸出金属,研究H2O2用量、H2O2加入速度、初始盐酸浓度、浸出温度、初始液固比和浸出时间对金属浸出率的影响。最终得到最优浸出条件为:H2O2用量0.8 mL/g(氧化还原电位为429 mV)、H2O2加入速度1.0 mL/min、初始硫酸浓度1.0 mol/L、初始盐酸浓度1.0 mol/L、浸出温度80 °C、初始液固比5:1 mL/g以及浸出时间1.5 h。在此最优条件下,铜冶炼烟尘中的铜和砷能被有效地浸出,剩下的浸出渣可作为一种合适的铅冶炼资源。此时,铜、砷和铁的平均浸出率分别为95.27%、96.82%和46.65%。
关键词:铜;冶炼烟尘;氧化浸出;电位
(Edited by Xiang-qun LI)
Foundation item: Project (2016M602427) supported by the Postdoctoral Science Foundation of China; Project supported by the Fundamental Research Funds for the Central Universities of Central South University, China
Corresponding author: Lin CHEN; Tel: +86-15111045540; E-mail: chenlin0210@csu.edu.cn
DOI: 10.1016/S1003-6326(18)64830-7

