钛铁矿(FeTiO3)粉末的氧化过程中的物相转变、微观形貌及其氧化机制
来源期刊:中国有色金属学报(英文版)2013年第8期
论文作者:肖 玮 鲁雄刚 邹星礼 危雪梅 丁伟中
文章页码:2439 - 2445
关键词:钛铁矿;物相转变;微观形貌;氧化机理
Key words:ilmenite; phase transitions; micro-morphology; oxidation mechanism
摘 要:探讨钛铁矿氧化过程中的物相转化、形貌改变及其氧化机理。在700~800 °C时,钛铁矿(FeTiO3)转变为赤铁矿(Fe2O3)和金红石(TiO2),当温度高于900 °C时,三价铁板钛矿开始形成。原始的钛铁矿粉末呈现顺磁性,但是经过中温(800~850 °C)氧化后,氧化产物呈现弱铁磁性。同时,讨论钛铁矿的氧化机理。通过对微结构的观察,发现在中温氧化过程中颗粒表面出现大量微孔,其在氧化过程中能够强化氧的传质。
Abstract: Phase transitions, morphology changes, and oxidation mechanism of the ilmenite oxidation process were investigated. FeTiO3 transforms to hematite and rutile when oxidation at 700-800 °C, and pseudobrookite is formed when the oxidation temperature reaches 900 °C. The initial ilmenite powder exhibits paramagnetism; however, after being oxidized at the intermediate temperature (800-850 °C), the oxidation product exhibits weak ferromagnetism. The oxidation mechanism was discussed. The microstructure observations show that a lot of micro-pores emerge on the surfaces of ilmenite particles at the intermediate temperature, which is deemed to be capable of enhancing the mass transfer of oxygen during oxidation.
Trans. Nonferrous Met. Soc. China 23(2013) 2439-2445
Wei XIAO, Xiong-gang LU, Xing-li ZOU, Xue-mei WEI, Wei-zhong DING
Shanghai Key Laboratory of Modern Metallurgy and Materials Processing, Shanghai University, Shanghai 200072, China
Received 21 June 2012; accepted 15 October 2012
Abstract: Phase transitions, morphology changes, and oxidation mechanism of the ilmenite oxidation process were investigated. FeTiO3 transforms to hematite and rutile when oxidation at 700-800 °C, and pseudobrookite is formed when the oxidation temperature reaches 900 °C. The initial ilmenite powder exhibits paramagnetism; however, after being oxidized at the intermediate temperature (800-850 °C), the oxidation product exhibits weak ferromagnetism. The oxidation mechanism was discussed. The microstructure observations show that a lot of micro-pores emerge on the surfaces of ilmenite particles at the intermediate temperature, which is deemed to be capable of enhancing the mass transfer of oxygen during oxidation.
Key words: ilmenite; phase transitions; micro-morphology; oxidation mechanism
1 Introduction
Ilmenite (FeTiO3) is the most abundant mineral which is the raw material for producing TiO2 and Ti. And it is one of the candidates for interesting electronics and spintronics materials [1,2]. Recent theoretical calculations predicted that the α-Fe2O3-FeTiO3 system had possibility to lead a magnetic semiconductor with strong spin-dependent transport properties and high Curie temperature of approximately 727 °C [3-6]. Moreover, FeTiO3 is a low-cost and promising oxygen carrier for solid fuels combustion in a chemical-looping combustion (CLC) system [7,8]. On the other hand, preoxidation is also a common pretreatment before subsequent reduction to separate Fe from the titanium oxides in many processes such as the industrial processes for producing synthetic rutile from FeTiO3 and some novel gaseous reduction processes [9-14].
Phase transition behaviors of FeTiO3 oxidation have been investigated by many researchers [15,16]. One of the main oxidation products is usually pseudobrookite when oxidation temperature is above 900 °C, which is stable phase at the high temperatures. Due to the different valence states of Fe, phase transitions occur during oxidation process. It is important to understand the phase transition process during oxidation and the phase composition in the oxidized products. At a lower temperature in the range of 500-800 °C, both rutile, hematite, and crystallographic shear (CS) structure phases are formed simultaneously during the oxidation of natural and synthetic FeTiO3 [17-19]. The CS phases were first discovered by KARKHANAVALA and MOMIN in an FeTiO3 sample heated at 650 °C in air [17]. GUPTA et al [20] reported that TiO2, hematite, and CS phase series were the stable products when the ilmenite sample was heated at the temperature lower than 850 °C.
In order to achieve a comprehensive understand about the oxidation behavior of FeTiO3, isothermal thermogravimetric (TG) experiments were carried out by a TG analyzer, and the phase compositions of products were determined by X-ray diffraction (XRD). The mechanism involved the oxidation pathway of the FeTiO3 was also discussed in this work.
2 Experimental
2.1 Preparation and characterization of synthetic FeTiO3
In this work, FeTiO3 was synthesized by a solid state reaction process. Fe2O3 and TiO2 powders were firstly mixed at a appropriate stoichiometric ratio corresponding to FeTiO3, and then the mixture was reacted at 1200 °C for 10 h, afterwards, the intermediate product was partially reduced by CO/CO2 (the volume ratio is 3:2) gaseous mixture in a static bed reactor. The sintered FeTiO3 was milled by a planet-type grinding mill. The crystal structure of the product (FeTiO3) was characterized by XRD. And the particle size distribution was measured by a laser particle analyzer. The morphology of the FeTiO3 was observed by a scanning electron microscopy (SEM).
2.2 Oxidation experiments
A horizontal furnace was used as a static bed reactor, and the synthetic FeTiO3 powder was spread in the bottom of a porcelain combustion boat. The gaseous mixture flowed through high-accuracy mass flowmeters, and then entered into the furnace chamber. The gaseous mixture was composed of pure O2 and high-purity Ar. When the temperature of the furnace reached the required temperature, ilmenite sample loaded in combustion boat was put into the furnace and oxidized for a prestted time.
2.3 TG-DSC thermal analysis
TG and DSC experiments were carried out by a simultaneous TG-DTA/DSC apparatus (STA 449 F3 Jupiter, Netzsch Ins.). The balance protective gas was high purity N2, and the purge gas was pure air. The exothermic and endothermic signals were measured by a platinum thermoprobe, and the gravimetric changing was detected by a high accuracy balance.
3 Results and discussion
3.1 Oxidation of ilmenite
Phase compositions of the initial material and the partial reduced products determined by XRD are shown in Fig. 1. As evidenced from Fig. 1 for synthetic ilmenite, the peak positions corresponding to (211),  (220) plane are 2θ=32.525, 35.258, and 48.722, respectively, therefore it is obvious that the FeTiO3 is formed.
(220) plane are 2θ=32.525, 35.258, and 48.722, respectively, therefore it is obvious that the FeTiO3 is formed.
TG-DSC plots of FeTiO2 oxidation are shown in Fig. 2. TG plot shows a slow increase in mass at 300- 800 °C, which is coincident with the main reaction temperature zone. A wide exothermic peak is found at a relatively low temperature range, and there is another exothermic peak at the temperature range of 600-900 °C. The initial FeTiO3 powder can be oxidized slowly below 600 °C, and the peak at 724.4 °C is corresponding to the formation of new phases. As shown in Fig. 3, no significant change is observed from room temperature to 500 °C, and the main phase is the initial FeTiO3.
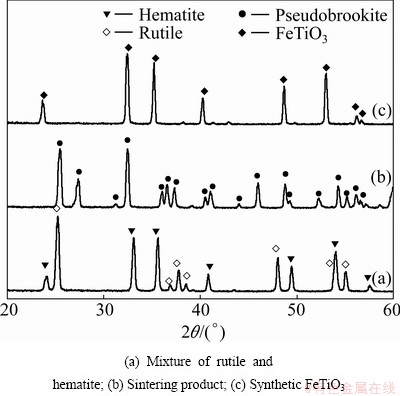
Fig. 1 XRD patterns of samples
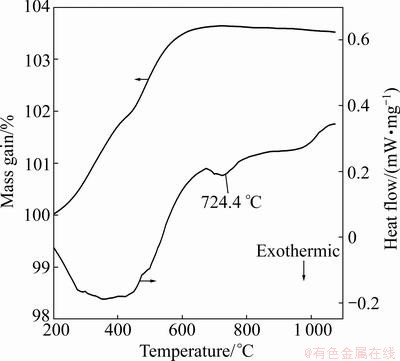
Fig. 2 TG-DSC plots of oxidation process of FeTiO3 powder
Isothermal oxidation experiments of the synthetic FeTiO3 sample were carried out at temperature range of 500-1000 °C. The phase transitions of FeTiO3 at different temperatures were analyzed by XRD. Figure 3 shows XRD patterns of the synthetic FeTiO3 oxidized at different temperatures in air for 2 h. At the temperatures of 500 and 600 °C, the diffraction peaks of initial synthetic ilmenite are strong, this may attribute to the slow reaction rate. The main phases of the oxidized product obtained from oxidation at 500 and 600 °C are FeTiO3 with a small amount of hematite and Fe2Ti3O9. At 700-800 °C, the main oxidized products are rutile (TiO2) and hematite (Fe2O3) with a small amount of Fe2Ti3O9. Above 900 °C, the main product is pseudobrookite, but coexists with a small amount of hematite and rutile.
Isothermal TG plots of oxidation obtained from different temperatures are shown in Fig. 4, from which the oxidation conversion rate can be determined. There is no detectable oxidation of large particle size FeTiO3 below 500 °C. The reaction rate is slow at lower temperatures and increases rapidly with rising temperature, especially above 800 °C. At 900 °C, the FeTiO3 samples can be completely oxidized within 30 min.
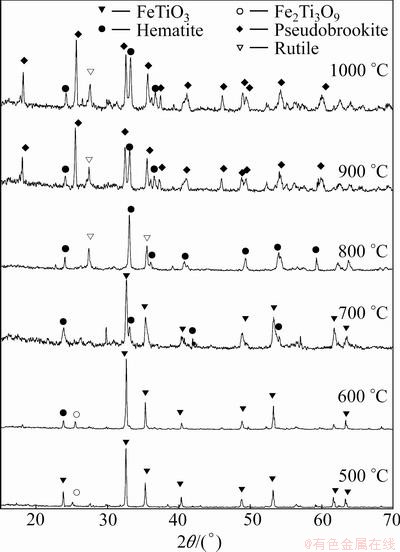
Fig. 3 XRD patterns of synthetic FeTiO3 oxidized at different temperatures in air for 2 h
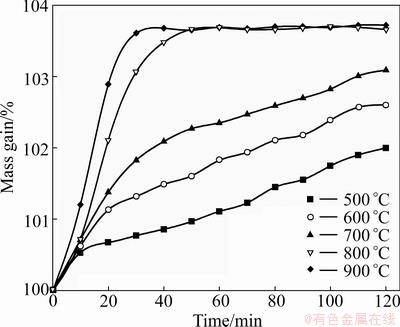
Fig. 4 Isothermal oxidation TG plots of FeTiO3 at different temperatures
3.2 Factors determining phase transitions at specific temperatures
3.2.1 Oxygen partial pressure
The oxygen partial pressure for the oxidation reaction was varied from 0.01 MPa to 0.1 MPa. The samples were oxidized at 600 °C for 30 min. The changes of the oxygen partial pressure did not affect the phase composition obviously. After 30 min oxidation, the main phases are also FeTiO3 and a small amount of rutile, but the diffraction peaks of Fe2Ti3O9 become stronger with the increase of oxygen partial pressure (see Fig. 5). The results indicate that the stability of Fe2Ti3O9 is enhanced with increasing O2 partial pressure.
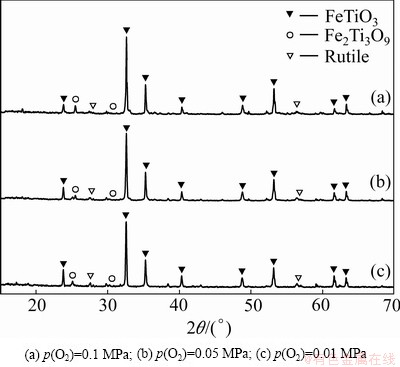
Fig. 5 XRD patterns of products obtained from oxidation under different oxygen partial pressures at 600 °C
3.2.2 Particle size of initial ilmenite
The oxidation rate of FeTiO3 with different particle sizes at 700 °C is shown in Fig. 6. Since the specific surface area increases with decreasing particle size, the oxidation rate increases in the row from samples (a) to sample (d), and the medium diameters (D50) of different samples are 21, 11, 5.6, and 0.76 μm, respectively.
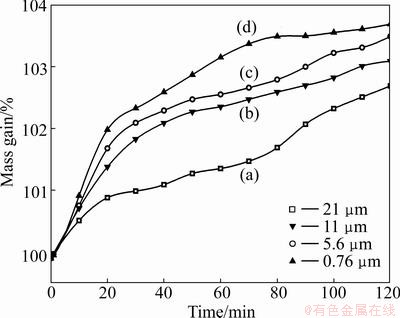
Fig. 6 Isothermal oxidation TG plots of ilmenite powders with different particle sizes
3.3 Effect of oxidation on magnetic property
Figure 7 shows the plots of magnetization (M) versus magnetic field (H) for the synthetic ilmenite and oxidation products measured at room temperature. The coercive field and magnetization of the initial FeTiO3 powder are around 0.01254 T and 0.00725 A/m (see Fig. 7(a)). It exhibits a paramagnetism, which reveals that the magnetic behavior of synthetic FeTiO3 powder is different from the bulk FeTiO3 (antiferromagnetism) [1,2,21]. The paramagnetic component could originate from the cation-disordered antiphase domain boundaries. The larger coercivity might result from the increase of the grain boundaries in the powder samples [22]. After being oxidized in air for 15 min, the sample exhibits a weak ferromagnetism, and its coercive field is 0.082199 T (see Fig. 7(b)). The main reason for transition of magnetism is the formation of hematite. And the magnetization is still not saturated at a high magnetic field (1.8 T), indicating a few of paramagnetic component is presented in the oxidized product. The sample changes into paramagnetism again after 2 h oxidation at 700 °C, although a very weak ferromagnetism is observed in the low magnetic field.
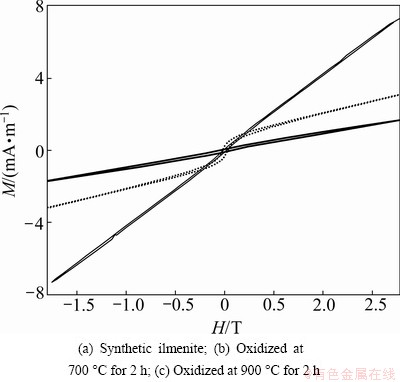
Fig. 7 Magnetization with magnetic field of different samples at room temperature
3.4 Oxidation mechanism of FeTiO3
The pathway of the phase transition during the oxidation of FeTiO3 is as follows:
4FeTiO3(s)+O2(g)→4TiO2(s)+2Fe2O3(s) (1)
A small amount of Fe2Ti3O9 is formed as the product of the parallel reaction:
12FeTiO3(s)+3O2(s)→4Fe2Ti3O9(s)+2Fe2O3 (2)
The rate of Eq. (1) is faster, and rutile and hematite are the main products in the relatively low temperature oxidation. On the other hand, the formation rate of Fe2Ti3O9 increases slightly with the increase of the oxygen partial pressure. The experiments are carried out in which rutile reacts with hematite in the temperature range of 600-700 °C, but Fe2Ti3O9 is not observed. By calculations of thermodynamics data which have been reported [24], Fe2Ti3O9 cannot be produced by the pathway described in Eq. (3). And its reverse reaction can occur, in other words, Fe2Ti3O9 may be an intermediate product which only exists in the relatively low temperature oxidation process.
3TiO2(s)+Fe2O3(s)→Fe2Ti3O9(s) (3)
When oxidation temperature reaches up to 900 °C, the crystal lattices of rutile, hematite and Fe2Ti3O9 disintegrate, in contrast, a new pseudobrookite crystal phase is formed. The pseudobrookite is the main stable phase at high temperatures (above 900 °C). Equations (4)-(6) may be responsible for the formation of pseudobrookite.
TiO2(s)+Fe2O3(s)→Fe2TiO5(s) (4)
Fe2Ti3O9(s)+2Fe2O3(s)→3Fe2TiO5(s) (5)
4FeTiO3(s)+2Fe2O3(s)+O2(g)→4Fe2TiO5 (6)
Fe2Ti3O9 is found to be stable below 900 °C in the experimental conditions, and it may play an important role in the transmission of O2-. It is suggested that iron is mobile toward the interface layer where it is oxidized [23]. The tentative mechanisms are shown schematically in Fig. 8. Hematite and rutile are formed during the oxidation of FeTiO3 below the temperature of 900 °C. O2 must approach the vicinity of the reaction layer and penetrate it, then, further reaction can take place at the interface of FeTiO3-Fe2O3.
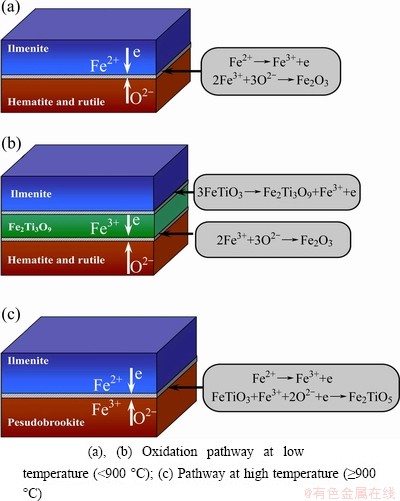
Fig. 8 Schematic representation of proposed mechanism for oxidation of FeTiO3
The diffusion rate of O2 is assumed to be low, and the solid-phase diffusion mechanism has been proposed by RAO and RIGAUD [15,24] to explain how ilmenite can be oxidized in the absence of pores for the mass transfer of the oxygen reactant. However, the oxidation rate at the intermediate temperatures becomes too rapidly to be explained by the solid-phase diffusion mechanism (Fig. 4).
SEM characterization was used to study the detailed morphology of the oxidation products. An interesting observation is that many micro-pores emerge on the surface of the FeTiO3 particles at moderate and high temperatures.
After being oxidized at 650 °C for 30 min, the surface of the sample changes greatly (Fig. 9(a)). A lot of clusters generate on the sample surface which has been reported as the formation of the ferric oxide layer [25-32]. The enrichment behavior of the ferric oxide on the particle surface and the morphology of this layer is similar to that occurring during the oxidation of Fe [25-30] and the cationic-selective oxidation of alloys [31,32]. If the temperature increases continuously, the irregular clustered morphology will disappear and the particle surface becomes rough (Fig. 9(b)). In the detailed observation, shown in Fig. 9(d), a large number of micro-pores which are deemed to be capable of enhancing the mass transfer rate of O2 in the oxidized reaction are created on the particle surface. The micro-pores less than 100 nm in diameter can be served as the mass transfer channels of O2. The O2 molecule can reach the vicinity of the reaction layer directly, therefore, the oxidized reaction can be completed in a short time. However, the porous structure is presented as an intermediate form. As the temperature increases, the micro-pores will be closed and a sintered structure appears on the surface of the particle (Figs. 9(c) and (e)).

Fig. 9 Typical morphology of sample surface
4 Conclusions
The phase transition of FeTiO3 oxidized by O2 is influenced significantly by the oxidation conditions. The oxidation products obtained in the temperature range from 500 to 800 °C comprise rutile, hematite, and Fe2Ti3O9. During the oxidation process at intermediate temperatures (800-850 °C), rutile and Fe2Ti3O9 are formed by parallel reactions. When temperature is above 900 °C, pseudobrookite is the only stable phase. With the phase transition, the magnetism of the oxidized product changes from paramagnetism to weak ferromagnetism, and then becomes paramagnetism again. The micro-morphology of the oxidized products changes greatly, at the relatively low temperatures (600-650 °C), cluster structure which is the formation of ferric oxide layer generates on the surface of the FeTiO3 particles. As the temperature increases, porous structure is observed, which may enhance the mass transfer of O2 and also is responsible for the rapid oxidation rate at intermediate temperatures.
References
[1] TANG Xin, HU Ke-ao. The formation of ilmenite FeTiO3 powders by a novel liquid mix and H2/H2O reduction process [J]. Journal of Materials Science, 2006, 41(23): 8025-8028.
[2] YE Fu-xing, OHMORI A. The photocatalytic activity and photo-absorption of plasma sprayed TiO2-Fe3O4 binary oxide coatings [J]. Surface and Coating Technology, 2002, 160(1): 62-67.
[3] FUJII T, KAYANO M, TAKADA Y, NAKANISHI M, TAKADA J. Ilmenite-hematite solid solution films for novel electronic devices [J]. Solid State Ionics, 2004, 172(1-4): 289-292.
[4] DAI Z, ZHU P, YAMAMOTO S, MIYASHITA A, NARUM K, NARAMOTO H. Pulsed laser deposition of ilmenite FeTiO3 epitaxial thin film onto sapphire substrate [J]. Thin Solid Films, 1999, 339(1-2): 114-116.
[5] ZHOU F, KOTRU S, PANDEY R K. Nonlinear current-voltage characteristics of ilmenite-hematite ceramic [J]. Materials Letters, 2003, 57(13-14): 2104-2109.
[6] FUJII T, KAYANO M, TAKADA Y, NAKANISHI M, TAKADA J. Preparation and characterization of epitaxial FeTiO3+δ films [J]. Journal of Magnetism and Magnetic Materials, 2004, 272-276: 2010-2011.
[7] BERGUERAND N, LYNGFELT A. Chemical-looping combustion of petroleum coke using ilmenite in a 10 kWth unit-high-temperature operation [J]. Energy Fuels, 2009, 23(10): 5257-5268.
[8] LEION H, LYNGFELT A, JOHANSSON M, JERNDAL E, MATTISSON T. The use of ilmenite as an oxygen carrier in chemical-looping combustion [J]. Chemical Engineering Research and Design, 2008, 86(9): 1017-1026.
[9] ZHANG G, OSTROVSKI O. Effect of preoxidation and sintering on properties of ilmenite concentrates [J]. International Journal of Mineral Processing, 2002, 64: 201-218.
[10] GUPTA S K, RAJAKUMAR V, GRIVESON P. Phase transformations during heating of ilmenite concentrates [J]. Chemistry and Materials Science, 1991, 22(5): 711-716.
[11] GUO Sheng-hui, LI Wei, PENG Jin-hui, NIU Hao, HUANG Meng-yang, ZHANG Li-bo, ZHANG Shi-ming, HUANG Ming. Microwave-absorbing characteristics of mixtures of different carbonaceous reducing agents and oxidized ilmenite [J]. International Journal of Mineral Processing, 2009, 93(3-4): 289-293.
[12] YANG Shao-li, SHENG Ji-fu. Smelting slag and pig iron technology [M]. Beijing: Metallurgy Industry Press, 2006: 12-16.
[13] GUO Yu-feng, LU Ya-nan, JIANG Tao, QIU Guan-zhou. Effect of pre-oxidation on Panzhihua ilmenite in solid state reduction process [J]. Journal of University of Science and Technology Beijing, 2010, 32(4): 413-419.
[14] WANG Yu-ming, YUAN Zhang-fu, GUO Zhang-cheng, TAN Qiang-qiang, LI Zhao-yi, JIANG Wei-zhong. Reduction mechanism of natural ilmenite with graphite [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(4): 962-968.
[15] RAO D B, RIGAUD M. Kinetics of the oxidation of ilmenite [J]. Oxidation of Metals, 1975, 9(1): 99-116.
[16] FU Xiao, WANG Yao, WEI Fei. Phase transitions and reaction mechanism of ilmenite oxidation [J]. Metallurgical and Materials Transactions A, 2010, 41(5): 1338-1348.
[17] KARKHANAVALA M D, MOMIN A C. The alteration of ilmenite [J]. Economic Geology, 1959, 54(6): 1095-1102.
[18] CHEN Ying. Low-temperature oxidation of ilmenite (FeTiO3) induced by high energy ball milling at room temperature [J]. Journal of Alloys and Compounds, 1997, 257: 156-160.
[19] CHEN Ying. Different oxidation reactions of ilmenite induced by high energy ball milling [J]. Journal of Alloys and Compounds, 1998, 266: 150-154.
[20] GUPTA S K, RAJAKUMAR V, GRIEVESON P. Phase relationship in the system Fe-Fe2O3-TiO2 at 700 and 900 oC [J]. Canadian Metallurgical Quarterly, 1989, 28(4): 331-335.
[21] CHEN Y H. Synthesis, characterization and dye adsorption of ilmenite nanoparticles [J]. J Non-Cryst Solid, 2011, 357: 136-139.
[22] YAN S, GE S, QIAO W, ZUO Y. Synthesis of ferromagnetic semiconductor 0.67FeTiO3-0.33Fe2O3 powder by chemical co-precipitation [J]. Journal of Magnetism and Magnetic Materials, 2010, 322(7): 824-826.
[23] SUN Kang, ZHANG Lei. Measurement of the thermodynamic properties of Fe2Ti3O9 and Fe2TiO5 [J]. Transactions of Nonferrous Metals Society of China, 1996, 6(1): 25-31.
[24] RAO D B, RIGAUD M. Oxidation of ilmenite and the product morphology [J]. High Temperature Science, 1974, 6(4): 323-341.
[25] TAKAGI R. Growth of oxide whiskers on metals at high temperature [J]. Journal of the Physical Society of Japan, 1957, 12: 1212-1218.
[26] GULBRANSEN E A, COPAN T P. Crystal growths and the corrosion of iron [J]. Nature, 1960, 186: 959-960.
[27] TALLAMN R L, GULBRANSEN E A. Dislocation and grain boundary diffusion in the growth of α-Fe2O3 whiskers and twinned platelets peculiar to gaseous oxidation [J]. Nature, 1968, 218: 1046-1047.
[28] VOSS D A, BUTLER E P, MITCHELL T E. The growth of hematite blades during the high temperature oxidation of iron [J]. Metallurgical Transactions A, 1982, 13A(5): 929-935.
[29] FU Y, CHEN J, ZHANG H. Synthesis of Fe2O3 nanowires by oxidation of iron [J]. Chemical Physics Letters, 2001, 350(5-6): 491-494.
[30] WEN Xiao-gang, WANG Su-hua, DING Yong, WANG Zhong-lin, YANG Shi-he. Controlled growth of large-are, uniform, vertically aligned arrays of α-Fe2O3 nanobelts and nanowires [J]. The Journal of Physical Chemistry B, 2005, 109(1): 215-220.
[31] KADIRI H E, MOLINS R, BIENVENU Y. Phase transformation and growth alumina on a thin FeCrAl-RE foil at around 900 °C [J]. Materials Science Forum, 2004, 461-464: 1107-1116.
[32] KADIRI H E, MOLINS R, BIENVENU Y. Abnormal high growth rates of metastable aluminas on FeCrAl alloys [J]. Oxidation Metals, 2005, 64(1-2): 63-97.
肖 玮,鲁雄刚,邹星礼,危雪梅,丁伟中
上海大学 上海市现代冶金与材料制备重点实验室,上海 200072
摘 要:探讨钛铁矿氧化过程中的物相转化、形貌改变及其氧化机理。在700~800 °C时,钛铁矿(FeTiO3)转变为赤铁矿(Fe2O3)和金红石(TiO2),当温度高于900 °C时,三价铁板钛矿开始形成。原始的钛铁矿粉末呈现顺磁性,但是经过中温(800~850 °C)氧化后,氧化产物呈现弱铁磁性。同时,讨论钛铁矿的氧化机理。通过对微结构的观察,发现在中温氧化过程中颗粒表面出现大量微孔,其在氧化过程中能够强化氧的传质。
关键词:钛铁矿;物相转变;微观形貌;氧化机理
(Edited by Chao WANG)
Foundation item: Project (51074105) supported by the National Natural Science Foundation of China; Project (51225401) supported by the China National Funds for Distinguished Young Scientists
Corresponding author: Xiong-gang LU; Tel: +86-21-56335768; E-mail: luxg@shu.edu.cn
DOI: 10.1016/S1003-6326(13)62752-1

