
Absorption of sulfur dioxide using membrane and enhancement of desorption with ultrasound
XUE Juan-qin(薛娟琴), LI Jing-xian(李京仙), LU Xi(卢 曦), MAO Wei-bo(毛维博),
WANG Yu-jie(王玉洁), WU Ming (吴 明)
School of Metallurgical Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, China
Received 6 July 2009; accepted 10 January 2010
Abstract: The absorption of sulfur dioxide in simulated flue gas by using liquid-containing membrane was investigated. The process of sulfur dioxide desorption from the absorbent of citrate solution was explored. The influence of the gas-phase, and the liquid-phase on absorption efficiency of sulfur dioxide and the influence of ultrasonic frequency, ultrasonic power and stirring speed on desorption efficiency of sulfur dioxide were examined. The results indicate that the absorption efficiency decreases with increasing flow velocity and sulfur dioxide content in gas-phase, and can be improved by increasing the concentration and the pH value of citrate solution. It is concluded that lower ultrasonic frequency results in a better degassing efficiency. The using of ultrasound in desorbing sulfur dioxide from citrate solution improves the desorbing efficiency in the some conditions, without changing the essence of chemical reaction.
Key words: sulfur dioxide; absorption; desorption; membrane; ultrasound; citrate; flue gas desulphurization
1 Introduction
Sulfur dioxide is one of the main components of the gas caused air pollution. Treatment of sulfur dioxide becomes important and imperative[1]. Currently, the absorption and desorption of sulfur dioxide by using citrate solution which was developed by Bureau Mineral Mountain Company[2] is one of the effective methods to control sulfur dioxide pollution. Membrane absorption is a new separation technique, which combines membrane with traditional absorption process[3-5]. Gas-phase is separated from liquid phase by the micropore in membrane and the transfer between the two phases is provided. The hollow fiber membrane is a kind of high polymer material with highly efficient separation function, which has been widely used in petrifaction, metallurgy, environment protection and food industry[6-12]. In this work, the membrane technique is combined with multi-element buffer solutions with the hollow fiber membrane chosen as the absorption equipment and citrate solution chosen as the absorption solution. The processes of the gas-phase and the liquid-phase have been studied comprehensively.
From the viewpoint of environmental protection and reasonable utilization of resources, it is necessary to desorb the citrate solution used for desulfurization. At present, this desorption is achieved mainly by steam heating in packing columns, which suffers from many drawbacks. The utilization of ultrasonic in sulfur dioxide degassing is a new method, which is simple in processing, overcomes some drawbacks of steam degassing and avoids the secondary pollution. As a new mass-transfer method, it has been researched intensively[13-16]. In this work, the steady cavitation in ultrasonic wave was used to make the gas dissolve in the absorbent, so sulfur dioxide can be desorbed and the absorbent can be recycled. A set of equipment for gas-liquid separation with high efficiency was designed and the influence of operating conditions on the degassing efficiency was studied. The results provide a technical way for the deep desorption of sulfur dioxide and the fundamental data for disposal of sulfur dioxide in future industry.
2 Theoretical analyses of absorption and desorption
The SO2 in gas and H2O react to produce H2SO3 when the flue gas containing SO2 goes through citrate solution, then the H+ from the dissociation and citrate acid radical combine to produce citrate acid. Because of the excellent buffer performance of the citrate solution, the H+ can be combined continuously, so the dissolvability of SO2 is enhanced in the citrate solution significantly.
The chemical reaction equations for the absorption and desorption of SO2 in citrate solution are
SO2(g)+H2O=H2SO3(aq) (1)
H2SO3(aq)=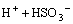 (2)
(2)
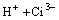 =
=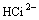 (3)
(3)
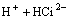 =
=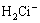 (4)
(4)
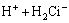 =
=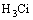 (5)
(5)
where Ci denotes citrate acid radical. The reactions go rightward for absorption and leftward for desorption. Therefore, the dominant contents in the citrate solution after the absorption of SO2 are H3Ci, HSO3- and H+. The distribution of the various citrate acid radical ions in citrate acid solution can be obtained by thermodynamic calculation[17].
Fig.1 shows the mass fractions of various citrate acid radical ions with different acidities. It is known from Fig.1 that citrate acid radical ions are simpler when they are at higher pH value.
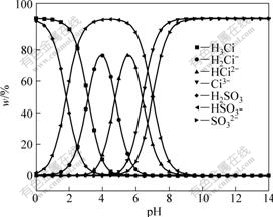
Fig.1 Distribution of various citric radical ions with respect to pH value of citrate solution
3 Absorption of sulfur dioxide with hollow fiber membrane
The simulated refining flue gas is chosen as the experimental gas, which is the mixture of air from an air compressor and a steel container with SO2, using high-precision gas-distribution system (φ(O2)>20%). The buffered sodium citrate is chosen as absorption liquid, which is of lab and analytical purity.
The modules of membrane are chosen to be made of PVDF (polyvinylidene fluoride), microporous hollow fiber membrane produced by Tianjin Motianmo Scientific and Technical Co. Ltd. and PP (polypropylene) membrane of d90 mm×1 106 mm produced by Zheda Kaihua Company, China.
The absorption efficiency of SO2 is defined as
η1=[(φ1-φ2)/ φ1]×100% (6)
where φ1 and φ2 are the SO2 contents before and after absorption, respectively.
3.1 Effect of gas-phase
3.1.1 Influence of flow velocity of gas-phase
The experimental conditions are as follows: SO2 content in gas is 0.27%, air pressure is 20 kPa, citrate solution pH is 4.50 and its concentration is 0.40 mol/L, absorption solution flow velocity is 55 L/h, circulation solution volume is 4.0 L, the option mode is chosen to be gas-liquid countercurrent, modules are horizontally laid, gas flows in tube, and absorption time is 6 h. The contents of SO2 in gas and solution were analyzed in a certain time interval. The results are shown in Fig.2.
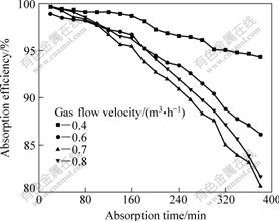
Fig.2 Influence of flow velocity of gas phase on absorption efficiency
Fig.2 shows that absorption efficiency is influenced by gas-phase flow velocity. By using the flow velocity of 0.4 m3/h, the absorption efficiency remains about 95% when the absorption time reaches 6 h, but with the flow velocity of 0.7 and 0.8 m3/h, it drops below 95% when absorption time reaches 3 h and drops to about 80% at 6 h. Furthermore, by using the flow velocity of 0.6 m3/h, it remains to be over 85% after 6 h. This behavior is in accord with the theoretical analysis. Because of the increase in the flow velocity of gas, the gas-phase mass-transfer resistance decreases and the total mass-transfer coefficient increases. But in reality, the absorption efficiency is not always increased with flow velocity of gas. Enhancing the flow rate of gas gives raise to increased energy consumption, shortens the time of the duration of gas staying in the module of membrane, and enhances the SO2 content at outlet, thus it decreases the absorption efficiency. So, the low-rate gas may lead to a high disposal efficiency and makes the SO2 content in outgoing flue gas satisfy the national effluent standard. But, a mini flow flux of gas will cause a problem for industrial disposal of a large amount of flue gas. Reducing the dispose amount per unit will lead to the increase of the number of membrane modules and the cost of disposal, so a suitable flow flux of gas should be chosen for optimization.
3.1.2 Influence of content of gas-phase
The experimental conditions are as follows: flow velocity of gas-phase is 0.6 m3/h and the other conditions are the same as Section 3.1.1. The concentrations of SO2 in gas and solution in a certain time interval were analyzed. The results are shown in Fig.3.
Fig.3 shows that the absorption efficiency drops while the sulfur dioxide content increases from 0.14% to 0.4% (volume fraction). The absorption efficiency is below 60% when the sulfur dioxide content reaches 0.4%. It is indicated that the absorption efficiency is much higher when the sulfur dioxide content in the gas is lower.
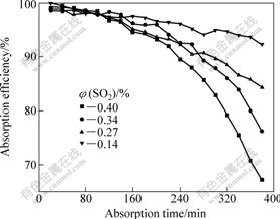
Fig.3 Influence of SO2 content on absorption efficiency
3.2 Effect of liquid-phase
3.2.1 Effects of concentration of absorption solution
The experimental conditions are as follows: citrate acid concentration is chosen to be 0.20, 0.40, 0.60 mol/L, respectively, the solution pH value is 4.5, SO2 content in gas is 0.27% (volume fraction). The results are shown in Fig.4.
Fig.4 shows that at the beginning of the absorption, the absorption efficiency is not sensitive to the concentration of absorption solution, but it becomes sensitive to the concentration when the absorption time is increased. A higher concentration of citrate results in a better absorption efficiency. Thus, enhancing citrate concentration is favorable to increasing the absorption rate and capability of solution. From reactions of Eqs.(3-5), it can also be found that citrate with a higher concentration is favorable to combining with H+, leading to a larger absorption efficiency. It is proved that the citrate solution has a strong absorption for SO2.
3.2.2 Effects of pH value of absorption solution
Theoretically speaking, SO2 is an acidic gas, thus, the increase of pH value of absorption solution is favorable to the absorption. In the experiment, a certain amount of sodium carbonate was added into the absorption solution in order to enhance the pH value of absorption solution. Fig.5 shows that the absorption with SO2 content of 0.27% (volume fraction) in incoming gas, citrate acid concentration of 0.60 mol/L and citrate pH values of 4.0, 5.0 and 6.0.
It is seen that the absorption efficiency with different pH values is very different when the absorption time increases. At 6 h, the absorption efficiency with pH 4.0 falls to about 55% while that with pH 6.0 remains above 95%. From the reaction (2), it is known that H+ is produced during the absorption of SO2. The decomposed H+ can be associated effectively by citrate acid radial at the beginning of absorption, so the absorption rate is not sensitive to the pH value. But, with the consumption of
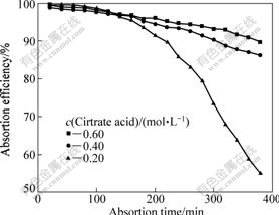
Fig.4 Effect of citrate concentration on SO2 absorption efficiency

Fig.5 Effect of solution acidity on SO2 absorption efficiency
citrate acid radial, the ability to associate H+ goes down for low pH absorption solution while the high pH solution maintains high ability to associate H+, thus it becomes sensitive to the pH value.
4 Desorption of sulfur dioxide with ultrasound
The prepared citric acid-sodium citrate buffer solution (θ=30 ℃, c=1.0 mol/L, pH=4.5) was allowed to absorb sulfur dioxide at a certain concentration used to provide the raw solution for testing the performance of the sonochemical reaction. In each experiment, 2.2 L of absorbent solution is filled into the sonochemical reactor, then the temperature-control system is employed to increase the liquid temperature to the set value. The ultrasonic system and the mixer are started to carry on the experiment of ultrasonic desorption, and monitor the concentration of sulfur dioxide in the solution.
The concentration of sulfur dioxide in the solution can be analyzed by the iodometric method. In this experimental system, the desorption efficiency in the absorbed sulfur dioxide is defined as
η2=[(φ1-φ2)/φ1]×100% (7)
where φ1 and φ2 are the contents of sulfur dioxide in the citrate solution before and after desorbing, respectively.
4.1 Influence of ultrasonic power
Ultrasonic power is defined as the total actual sound energy radiated into the reaction system in unit time. Only when the ultrasonic power is input into the solution, above the threshold value of cavitation can the cavitation effects occur. The ultrasonic degassing process mainly utilizes the steady cavitation, which can occur under the ultra-low sound strength, meaning that under the continuous action of sound wave, the bubbles in the liquid keep growing until rising to the surface of liquid and merging into the space. When the strength of the wave is very strong, the cavitation happens too quickly to make the bubbles rise to the liquid surface, so the sulfur dioxide re-dissolves in the citrate solution. Therefore, it is necessary to choose a suitable ultrasonic power for the desorption of sulfur dioxide.
It is known that the ultrasonic power is most sensitive to the reactor output power, which is scaled by the ultrasonic generator output power. Taking the actual conditions into consideration, 130, 175, 220, 255, 300, 340 and 370 W were chosen for the examination of the influence of output power on the desorption efficiency. The results are shown in Fig.6.
Fig.6 suggests that the desorption efficiency increases with the increase of the power at the beginning, then decreases, and the maximal value reaches 300 W. When the generator input power is above 300 W, the desorbing efficiency decreases with the power increasing. Therefore, the best electric power is 300 W at 40 kHz, and it is chosen in the experiments mostly.
4.2 Influence of ultrasonic frequency
With the same conditions as those in Section 4.1, the sulfur dioxide desorbing experiment is conducted with different ultrasonic frequencies (20, 40 and 60 kHz). The results in Fig.7 show that the sulfur dioxide desorption efficiency gets lower when the frequency gets higher. As the frequency goes up, the time of ultrasonic wave inflation becomes shorter and the cores do not have enough time to grow up to cavitation bubbles. Therefore, a lower frequency results in a better ultrasonic desorption. Furthermore, the threshold of cavitation is increased, when the cavitation is under a higher frequency. But, the ultrasonic waves with high frequency are more quickly dissipated in the solution and thus, more energy consumption is needed in order to obtain the same sonochemical effect. So, the ultrasonic with low frequency is beneficial in the aspect of energy consumption.
4.3 Influence of stirring speed
Fig.8 (the conditions are the same as in Section 4.1
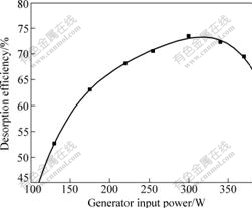
Fig.6 Relationship between desorption efficiency and input electric power
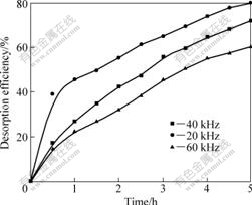
Fig.7 Relationship between desorption efficiency and ultrasonic frequency
except the stirring speed) suggests that the sulfur dioxide desorption efficiency increases with the stirring rate whether using the ultrasound or not. It increases slowly, when the stirring rate is above 250 r/min. This means that the stirring can speed up the gas diffusion and thus speed up the escape of the sulfur dioxide bubbles. Increasing the stirring rate can also enhance the cavitation effect. It is noted that the influence of stirring rate on the sulfur dioxide desorption is independent of the using of the ultrasound. Under the same stirring rate, the using of ultrasound can enhance desorption efficiency by 25%. So, the ultrasound just accelerates the desorption, without changing the mechanism of the desorption process.
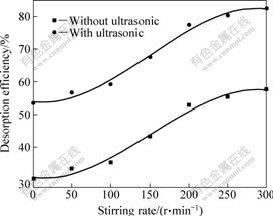
Fig.8 Relationship between desorption efficiency and stirring rate
5 Conclusions
1) The absorption efficiency can be lowered by increasing the flow velocity and SO2 content in gas phase. However, it can be enhanced by using the high citrate concentration. The absorption is sensitive to the pH. The higher pH the citrate solution has, the larger the absorption efficiency is.
2) Under some conditions, the using of ultrasonic can enhance the desorption without changing the reaction mechanism. At a frequency of 40 kHz for ultrasonic, desorption temperature of 50 ℃, generator output power of 300 W, stirring rate of 300 r/min, citrate concentration of 1.0 mol/L, initial SO2 concentration of 120 g/L and reaction of 5 h, a desorption efficiency of 82% can be obtained.
References
[1] XIAO Wen-de, WU Zhi-quan. Removing and reuse of sulfur dioxide [M]. Beijing: Chemistry Technology Press, 2001: 2-10. (in Chinese)
[2] NISSEN W, CROCKER L. Citrate process for flue gas desulfurization [J]. Bull US Bur Mines, 1985, 68(6): 75-82.
[3] MULDER M. Principles of membrane technology [M]. Beijing: Tsinghua University Press, 1996: 243-247. (in Chinese)
[4] SONG Xue-yan, MENG Fan-gang, YANG Feng-lin. Application of seawater to enhance SO2 removal from simulated flue gas through hollow fiber membrane contactor [J]. Journal of Membrane Science, 2008, 312: 6-14.
[5] LIN Li-gang, KONG Ying, WANG Gang. Selection and crosslinking modification of membrane material for FCC gasoline desulfurization [J]. Journal of Membrane Science, 2006, 285: 144-151.
[6] NOHREN J, MIERAU B, SMITH T, MCLENNAN R. Hollow fiber membrane filters [J]. Membrane Technology,2003, 5:12-16.
[7] YEN Hsing-yuan, YANG Mu-hoe. Modified solution-diffusion model analysis of the flue gas desulfurization effluents in a polymide membrane [J]. Polymer Testing, 2003, 22: 109-113.
[8] ZHAN Han-hui, ZHANG Jing-jing, LUO Ding-ti. Reducing concentration polarization in hollow-fibre membranes [J]. Membrane Technology, 2004,6: 5-9.
[9] QI Rong-bin, WANG Yu-jun, LI Ji-ding. Pervaporation separation of allkane/thiophene mixtures with PDMS membrane [J]. Journal of Membrane Science, 2006, 280: 545-552.
[10] KALDIS S P, KAPANTAIDAKIS G C, SAKELLAROPOULOS G P. Simulation of multicomponent gas separation in a hollow fiber membrane by orthogonal collocation—Hydrogen recovery from refinery gases [J]. Journal of Membrane Science, 2000, 173: 61-71.
[11] LIN Li-gang, WANG Gang, QU Hui-min. Prevaporation performance of crosslinked polythlene glycol membranes for deep desulfurization of FCC gasoline [J]. Journal of Membrane Science, 2006, 280: 651-658.
[12] LAURA W L, WILLIAM J F, BRACK G H. Gas permeability of hollow fiber membranes in gas-liquid system [J]. Journal of Membrane Science, 1996, 117: 207-219.
[13] WANG You-le, ZHUO Jun, XIE Gang. The research on the management of high concentration ammonia nitrogen waste water by ultrasonic wave technique [J]. Technique and Equipment for Environmental Pollution Management of Chinese, 2000(2): 59-63. (in Chinese)
[14] GONDREXON N, RENAUDIN V, BOLDO P. Degassing effect and gas-liquid transfer in a high frequency sonochemical reactor [J]. Chemical Engineering Journal, 1997, 66: 21-26.
[15] ESKIN G I. Cavitation mechanism of ultrasonic melt degassing [J]. Ultrasonics Sonochemistry, 1995(2): 137-141.
[16] XUE Juan-qin, FAN Rui-jiang, WANG Zhao-qi. Application of sonochemical reactor in SO2 removing process [J]. Chemistry, 2006, 69(1): 52-56. (in Chinese)
[17] XUE Juan-qin, WANG Kong-fen, YANG Juan-juan. Mechanism of gas desulfurization with citrate solution [J]. Journal of Chemical Industry of Engineering(China), 2008, 59(4): 1022-1027. (in Chinese)
(Edited by YANG Bing)
Foundation item: Projects(50874087, 50978212) supported by the National Natural Science Foundation of China
Corresponding author: XUE Juan-qin; Tel: +86-13186038833; E-mail: huagong1985@163.com
DOI: 10.1016/S1003-6326(09)60238-7