
Effect of T4 and T6 treatment on corrosion of die cast AZ91D magnesium alloys in 3.5% NaCl
ZHOU Wan-qiu(周婉秋)1,2, SHAN Da-yong(单大勇)1 , HAN En-hou(韩恩厚)1 , KE Wei(柯 伟)1
1. Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China;
2. College of Material Science and Engineering, Beijing University of Chemical Technology, Beijing100029, China
Received 28 July 2006; accepted 15 September 2006
Abstract: The effect of heat treatment on microstructure and corrosion behavior of die-cast AZ91D magnesium alloys in 3.5% NaCl solution was investigated by SEM, EDX, XRD and electrochemical technique. It is found that the distribution of β phase influences the corrosion morphology. Corrosion occurs preferentially in primary α phase and presents pitting corrosion feature in die-cast AZ91D. After homogenization of T4 treatment, β phase dissolves in α phase and forms a single phase with α matrix, and the corrosion form turns to localized corrosion. The attack initiates at local site, expands towards deep direction and produces “digging effect”. After artificial aging of T6 treatment, β phase is produced in abundance and provides a great deal of effective micro-cathode for anodic dissolution, and the corrosion form exhibits in general corrosion.
Key words: AZ91D magnesium alloys; microstructure; corrosion behavior
1 Introduction
There is growing interest in magnesium alloys in the automotive industry and electronic products[1]. Die-casting is one of the most commonly used methods of producing magnesium alloy component because of its capability of a high production rate of high quality parts at a reasonable cost. Cast magnesium alloys are prone to suffer pitting attack when exposed in corrosive service environment especially containing chloride[2-3].
Although a few studies have been reported earlier on the effect of microstructure on the corrosion behavior of AZ91 alloys[4-6], few work was focused on corrosion form which was caused by second phase distribution. The present investigation is concentrated on studying the effect of heat treatment on microstructure and corrosion behavior of AZ91D alloys prepared by die-cast. The experimental results exhibit that the corrosion form is related to the distribution of β phase.
2 Experimental
Die-cast AZ91D plates were used with the composition of 8.3%-9.7%Al, 0.35%-1.0%Zn, 0.15%- 0.50% Mn, <0.1%Si, <0.03%Co, <0.002%Ni, <0.005%Fe, and balance Mg. Solution treatment (T4) was carried out by keeping die-cast AZ91D specimen at 415 ℃ for 10 h under the protection of flowing high purity argon, followed by quenching in water. Aging treatment(T6) was conducted by keeping the T4 treat- ment specimen at 216 ℃ for 6 h under the protection of flowing high purity argon and cooling naturally to room temperature. In order to locate the preferentially corroded sites on a micro-scale, metallographic samples after slight etching were immersed into the test solution for a few minutes, rinsed with water and then alcohol, dried by flowing air, and observed with SEM. Metallurgical specimens were prepared by polishing with 1 200 grit SiC paper and 1 μm diamond paste, and then were etched for about 2 s in 5% nital solution. The samples for electrochemical measurement were molded into epoxy resin with only one side exposed as working surface available. Electrochemical tests were carried out using EG & G M273 potentiostat with a saturated calomel electrode as reference electrode and platinum flake as auxiliary electrode.
EIS measurements were conducted using EG & G M273 potentiostat coupled with M5210 lock-in amplifier with a perturbing signal of AC amplitude of 5 mV and a frequency ranging from 100 kHz to 5 mHz. The testing solution was prepared with AR grade NaCl in distilled water and saturated with Mg(OH)2 to keep pH value at 11 during the measurement. Morphology was observed by SEM. Chemical composition was analyzed by EDX.
3 Results and discussion
3.1 Microstructure and corrosion morphology
The microstructure of die-cast AZ91D magnesium alloys present a typical network structure with the primary α phase and the eutectic phase consisting of eutectic magnesium and the second phase β. β phase is a metallic compound of Mg17Al12 which distributes along the grain boundary and forms network precipitation, as shown in Fig.1(a). Fig.1(b) reveals the initial corrosion morphology of die-cast specimen in 3.5% NaCl solution. Corrosion takes place preferentially in α phase, and no obvious attack emerges on β phase. Fig.1(c) illustrates the morphology of die-cast AZ91D after 6 h exposure. Corrosion cracks occur and corrosion product deposits on local surface, however, most of the specimens do not break. EDX result indicates that the corrosion product contains Mg 43.6%, Al 9.72%,O 44.85% and Cl 1.83%. XRD analysis indicates that the corrosion product is mainly composed of Mg(OH)2, alumina, aluminum hydroxide and chlorine comes from the corrosive medium.

Fig.1 Morphologies of AZ91D magnesium alloys: (a) Die-cast; (b) Initial corrosion of die-cast; (c) Die-cast after 6 h exposure; (d) Die-cast after T6 treatment; (e) Initial corrosion of T6 specimen; (f) T6 specimen after 48 h exposure; (g) Die-cast after T4 treatment; (h) T4 specimen after 48 h exposure
After aging treatment T6, abundant β phase is produced and spreads all over specimen. Slice-shape β phases are parallel to each other or presents radiation shape, α phase exists among β slices as shown in Fig.1(d). Fig.1(e) illustrates the image of etched T6 specimen after 1 h immersion in 3.5% NaCl solution. Corrosion develops very quickly and spreads all over the surface in initial time. It can be found that corrosion occurs in α phase and the trace of β phase can also be observed. Corrosion on T6 specimen takes place in a short time and extends all over surface rapidly. General corrosion develops on entire surface with time. After 48 h immersion, a great amount of corrosion product deposits on metal surface and exhibits general corrosion feature as illustrated in Fig.1(f).
After T4 homogenization treatment, β phase dissolves in metal matrix and forms a single phase solid solution as presented in Fig.1(g). The EDX result indicates that aluminum distributes homogeneously in metal matrix.
For T4 treatment specimen, attack takes place at local site and hydrogen generates intensively from the pitting. The metal surface keeps shining appearance outside the pitting. The pitting sites increase with time increasing, the pitting area is enlarged and the pit depth extends as shown in Fig.1(h).
3.2 OCP measurement
Fig.2 presents the open circuit potential(OCP) vs time curves for AZ91D under different conditions in 3.5%NaCl solution. For die-cast AZ91D, OCP shifts positively in initial time and stabilizes at about -1.54 V. For T6 specimen, the potential moves towards positively in initial immersion and tends towards -1.56 V with time prolonging. For T4 specimen, OCP attains a maximum of -1.54 V rapidly and then reduces gradually, and OCP keeps a relatively low value for a period of time and then shifts positively.
The variation of OCP reflects the change of electrode surface to some extent. Die-cast AZ91D takes place pitting corrosion, magnesium is dissolved in anode and hydrogen is reduced in cathode. Corrosion reaction reaches stable with time and OCP attains a comparatively constant value.
For T6 specimen, bulk fraction of β phase is much larger than that of die-cast specimen. SONG et al[7] indicated that β phase is inert in Cl- containing medium with a potential of about -1.0 V(vs SCE), which is obviously higher than α phase. β phase acts as effective cathode, which facilitates corrosion reaction and results in the decrease of OCP in initial time. The corrosion product covers metal surface with time and forms space barrier against further attack, which results in potential rise. Abundant β phase provides barrier for corrosion, which hinders the transportation of species concerning reaction and suppresses corrosion.

Fig.2 OCP vs immersion time in 3.5%NaCl solution for different samples
After T4 treatment, the aluminum content in metal matrix increases, and corrosion performance is improved to a certain extent. OCP attains a relatively high value in the initial time. However, pitting is produced on local site and expands deeply with time, which results in the negative movement of potential. Overlaying of corrosion product provides protection for metal substrate which causes potential positive movement with time.
3.3 EIS measurement
In order to understand the influence of heat treatment on corrosion behavior of die-cast AZ91D, the Nyquist plots of die-cast AZ91D, T4 and T6 specimens for different immersion time were measured using EIS technique. Fig.3 shows the EIS spectrum of die-cast AZ91D in 3.5%NaCl solution. Large capacity loop exists at high frequency, which refers the resistance of charge transfer in double layer. A small capacity loop occurs at low frequency, which is related to hydrogen evolution procedure. In the fourth quadrant, an inductive loop appears at very low frequency, which may be correlative to corrosive dissolution and adsorption of medial product produced during the process. It can be seen that the capacity loop shrinks with immersion time, which indicates the corrosion propagation with time. On the contrary, EIS of T6 specimen increases with time and attains a constant value as illustrated in Fig.4. In initial stage, impedance is very small and evident inductive loop appears. For T4 specimen, high frequency loop shrinks to large extent after 6 h immersion, and no distinct variety occurs after 18 h immersion as presented in Fig.5.
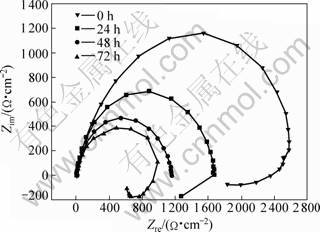
Fig.3 EIS spectrum of die-cast AZ91D in 3.5% NaCl solution for different times

Fig.4 EIS spectrum of die-cast AZ91D-T6 specimen in 3.5% NaCl solution for different times
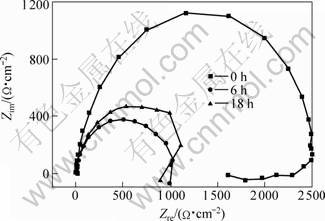
Fig.5 EIS spectrum of die-cast AZ91D-T4 specimen in 3.5% NaCl solution for different times
Die-cast AZ91D is a double phase alloy, which is made up of α and β phases. α phase has high chemical activity compared with β phase and is prone to occur corrosion dissolution. In the initial exposure time in 3.5% NaCl solution, α phase acts as anode, magnesium is dissolved and Mg2+ is produced, which results in the obvious diminishing of impedance. β phase acts as cathode, hydrogen ion is deoxidized at β phase and hydrogen gas is separated out, then OH- is produced and solution pH is increased. Mg2+ combines with OH- and Mg(OH)2 is produced. Corrosion product accumulates on metal surface gradually, which acts as barrier for further attack. α phase is dissolved preferentially. β phase remains in framework shape on metal surface, prevents the transformation of particles involved in corrosion reaction on metal surface and blocks the corrosion reaction. Dissolution of α phase originates from the centre part of α phase interior and extends towards β phase gradually. The corrosion for die-cast AZ91D shows pitting characteristic. The impedance decreases slightly and tends to stable value gradually.
A great deal of β phase are separated out after T6 treatment, and β occupies large volume fraction in alloys. As cathode for corrosion reaction, β provides a large reaction field for hydrogen reduction. It accelerates the anodic dissolution and leads to corrosion reaction swift at initial stage. Mg2+ ions have no enough time to diffuse completely to bulk solution due to the rapid reaction rate, which causes the enrichment of Mg2+ ion in the thin liquid layer near metal surface. The hydrogen evolution at β phase produces abundant OH- at metal/solution interface in a short time. The corrosion product mainly composed of Mg(OH)2 is deposited on metal surface and provides protection for metal substrate. α phase located on metal surface is dissolved off and β phase is reserved, so the corrosion barrier made of β phase is formed under corrosion product film, which results in impedance increase. When the impedance reaches a certain value, it attains stable state and does not change any more.
T4 treatment causes β disappearance, and aluminum element tends to distribute uniformly in metal matrix with a mass fraction of 8%-9%. No uniform effective cathode exists on metal surface after T4 treatment, so corrosion is unable to develop uniformly. In die-cast process, gas remains in alloy interior and is heated and expanded during T4 treatment, which results in skin metal dropping off at local sites and some scattered small holes are produced on metal surface. These small pits become feeble location in corrosive medium, and Cl- ions adsorb preferential at these lacunas. Once attack is from these positions, large cathode versus small anode corrosion micro-cell would be formed on metal surface. The substrate at pit is a small anode, so the corrosion current highly concentrates at the pit and drastic anodic dissolution occurs. The hydrogen evolution at cathode is also radical. The severe dissolution of substrate produces digging effect and expands towards depth direction, and high frequency capacity loop in impedance spectrum shrinks greatly. Pitting itself possesses cathode protective effect for other part of metal, so metal surface outside the pitting is not corroded and maintains metal shining.
4 Conclusions
1) Die-cast AZ91D is double phase alloy which is composed of α phase and β phase. β phase distributes at grain boundary and forms net work discontinuous precipitation. Corrosion initiates from α phase, and the corrosion form for diecast AZ91D is pitting.
2) After T4 treatment, β phase dissolves in metal matrix completely and alloy becomes single phase. Attack occurs only on some location in chloride containing medium, and the corrosion form is localized corrosion. Once pitting takes place, severe dissolution of substrate produces digging effect, and corrosion expands towards depth direction with abundant hydrogen emission.
3) After T6 treatment, a great deal of β phase is separated out, and abundant effective cathodes are provided for anodic dissolution, so corrosion occurs quickly and extends all over surface. The corrosion form is general corrosion.
References
[1] DECKER R F. The renaissance in magnesium[J]. Advanced Materials & Processes, 1998, 9: 31-33.
[2] MAKAR G L, KRUGER J. Corrosion of magnesium[J]. Int Mater Rev, 1993, 38(3): 138-145.
[3] SONG G L, ATRENS A. Corrosion mechanisms of magnesium alloys[J]. Advanced Engineering Materials, 1999, 1: 11-33.
[4] SONG G L, ATRENS A, DARGUSCH M. Influence of microstruc- ture on the corrosion of die-cast AZ91D[J]. Corrosion Science, 1999, 41: 249-273.
[5] ISHIKAWA K, KOBAYASHI Y. Precipitated structures and mechanical properties of AZ91D magnesium alloy[J]. J Japan Inst Metals, 1997, 61(10): 1031-1036.
[6] AMBAT R, AUNG N N, ZHOU W. Evaluation of microstructure effect on corrosion behavior of AZ91D magnesium alloy[J]. Corrosion Science, 2000, 42: 1433-1455.
[7] SONG G L, ATRENS A, WU X L. Corrosion behavior of AZ21, AZ501 and AZ91 in sodium chloride[J]. Corrosion Science, 1998, 40(10): 1769-1791.
(Edited by YUAN Sai-qian)
Foundation item: Project (2002AA331120) supported by the Hi-tech Research and Development Program of China
Corresponding author: ZHOU Wan-qiu; Tel: +86-24-23893115; E-mail: wqzhou@imr.ac.cn