
Nickel boride alloys as catalysts for successive hydrogen generation from sodium borohydride solution
WU Chuan(吴 川)1, 2, BAI Ying(白 莹)1, 2, WU Feng(吴 锋)1, 2, WANG Guo-qing(王国庆)1, 2
1. School of Chemical Engineering and the Environment, Beijing Institute of Technology, Beijing 100081, China;
2. National Development Center for High Technology Green Materials, Beijing 100081, China
Received 15 July 2007; accepted 10 September 2007
Abstract: Nickel boride alloys, Ni-B, were prepared using chemical reduction method by the reaction of metal chloride with sodium borohydride, and heat-treated at various temperatures. The structures of the as-prepared alloys were studied using X-ray diffractometry (XRD), scanning electronic microscopy (SEM) and nitrogen adsorption-desorption analysis. When being adopted as the catalysts for successive hydrogen generation from sodium borohydride solution, the Ni-B alloy treated at 90 ℃ achieves a maximum hydrogen generation rate of 15.4 L/(min?g), and an average hydrogen generation rate of 13.6 L/min, which can give successive hydrogen supply to a 2.2 kW PEMFC at a hydrogen utilization of 100%.
Key words: proton exchange membrane fuel cell; hydrogen generation; Ni-B alloy; catalyst
1 Introduction
With the rapid development of proton exchange membrane fuel cells (PEMFC), which adopts pure hydrogen as the best fuel, hydrogen generation and supply methods become one of the crucial issues in practice. Therefore, novel hydrogen generation techniques have attracted more and more attention[1], among which the hydrolysis of NaBH4 becomes a promising one. Since 1950s, SCHLESINGER et al[2] found that sodium borohydride could be adopted as a reducing agent in the generation of hydrogen. Hydrogen releasing from NaBH4 solution needs efficient catalysts for getting satisfied H2 generation rate. In 1962, BROWN et al[3] found platinum, ruthenium and rhodium slats had highly catalytic activities for NaBH4 hydrolysis. In recent years, ion exchange resin supported Ru[4], carbon supported Pt[5-6], and oxides supported Pt[7-8] have been adopted as efficient catalysts, and some catalysts based on transition metals[9-10] are also taken into account. A novel kind of catalysts, metal borides[11-14], are recently found to be cheap and effective accelerator, and can give satisfied hydrogen generation performances.
In this study, Ni-B alloys were prepared using chemical reduction method and followed by heat-treatment. The structural evolutions and the catalytic activities of the as-prepared Ni-B alloys were discussed and compared.
2 Experimental
2.1 Sample synthesis
Ni-B alloys were prepared using chemical reduction method by the reaction of metal chloride with sodium borohydride, which is similar to the synthesis of Co-B alloys[11]. The precipitates were carefully washed with deionized water to remove the soluble ions, and then vacuum-dried at 90 ℃ to get rid of residual water and hydrogen. The as-prepared precursor was heated in a nitrogen atmosphere at various temperatures to get various Ni-B alloys.
2.2 Structural characterization
Powder XRD patterns of all the as-prepared Ni-B alloys were recorded by Rigaku X-3000 X-ray powder diffractometer with Cu Kα radiation. The scan range was from 20? to 60?, and the scan rate was 5 ℃/min in step of 0.02?.
The morphologies of the Ni-B alloys were observed using JSM-35C scanning electron microscope (SEM).
Nitrogen adsorption-desorption of the Ni-B alloys were measured at -196 ℃ with an ASAP 2010 surface area analyzer (Micromeritics Instrument). Prior to the measurements, the catalysts were vacuum-dried for 12 h. The specific surface areas of the catalysts were determined from the N2 adsorption-desorption isotherms by the BET method.
2.3 Catalytic activity tests
Catalytic activity tests were performed with a hydrogen generation reactor. First of all, 100 mg Ni-B alloy was placed in the reactor. Subsequently, the reactor was sealed, and pure nitrogen gas was fed to drive the air away from the reactor. After that, 10% NaBH4- 5%NaOH solution was pumped with a feeding rate of 10 mL/min successively; the as-generated hydrogen was imported into a surge flask to wash out the residual alkali and water, and then passed through a flow meter.
3 Results and discussion
3.1 XRD patterns
Fig.1 shows the XRD patterns of the as-prepared Ni-B alloys. It is clear that the Ni-B alloy vacuum-dried at 90 ℃ shows an amorphous structure, which has only a broad peak from 35? to 55?. However, when heating temperature is 350 ℃, Ni3B is detected, which is different from Ni2B reported by CHLESINGER[15]. Additionally, Ni-B alloy becomes a mixed phase of Ni3B and metal Ni. When temperatures are 400 ℃ and 500 ℃, the diffraction peaks of Ni-B alloys become more and more pointed, and finally metal Ni is the dominant phase.
Compared with Co-B alloys, in which metal Co becomes the dominant phase only on condition that the heating temperature exceeds 500 ℃[11], the Ni-B alloys in this study decompose at relative low temperature. Since the molar fraction of B in Ni3B is only 25%, which is lower than 33%, there are six Ni atoms surround one B atom, and no strong B—B bonds can be formed. Thus, Ni-B alloys can easily decompose at 350 ℃, and form metal Ni.
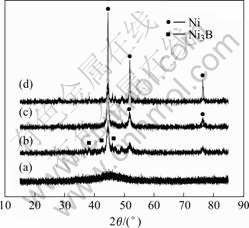
Fig.1 XRD patterns of Ni-B alloys heat-treated at various temperatures: (a) 90 ℃; (b) 350 ℃, (c) 400 ℃; (d) 500 ℃
3.2 SEM images
The apparent morphologies of the as-prepared Ni-B alloys are shown in Fig.2. The Ni-B alloy vacuum-dried at 90 ℃ shows non-uniform particle size, and floccular fragments are found. When heating temperature increases, the morphology of Ni-B alloy has no obvious change. However, large particles more than 20 μm are formed at 400 ℃, and grow larger with the increase of the heating temperature. This implies that with the increase of temperature, the Ni-B particles aggregate, and the structures of Ni-B alloys change from amorphous phases to crystal phases. These results are correspondent with those of XRD patterns in Fig.1.
3.3 BET analysis
The nitrogen adsorption-desorption isotherms of Ni-B alloys show typical type-III isotherms, which presents non-porous structures, as shown in Fig.3. The evolutions of BET surface areas of the Ni-B alloys treated at various temperatures are compared in Fig.4. It is found that the surface area of Ni-B alloy decreases with the increase of heating temperature.
When heating temperature increases from 90 ℃ to 400 ℃, the BET surface area decreases from 32 m2/g to 21 m2/g. This indicates that even the heating temperature increases more than 300 ℃, the BET surface areas of Ni-B alloys are still in the same order. However, when heating temperature increases from 400 ℃ to 500 ℃, the BET surface area decreases from 21 m2/g to 1.7 m2/g. This implies that the crystallization of Ni-B alloys is very violent between 400 ℃ and 500 ℃.
3.4 Hydrogen generation test
Fig.5 shows the relationship between hydrogen generation volume and reaction time. It is evident that the Ni-B alloys heat-treated at 90 ℃, 350 ℃ and 400 ℃ have similar catalytic activities, among which the one prepared at 90 ℃ shows the best performance, and achieves a hydrogen generation volume over 24 L after 18 min. The Ni-B alloy prepared at 500 ℃ shows almost no catalytic activity, and gets a hydrogen generation volume only 1.3 L after 18 min. Actually, metal atoms often serve as the activity centers in transition metal borides, and Ni-B alloy will decompose to form metal Ni at elevated temperatures, especially at
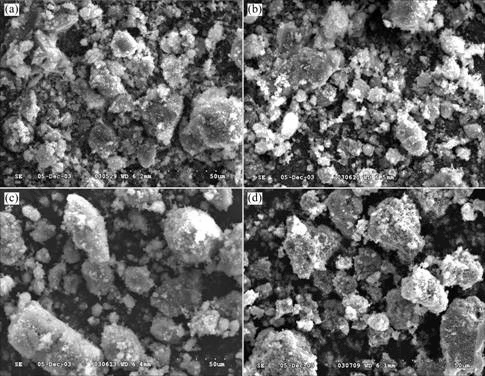
Fig.2 SEM images of Ni-B alloys heat-treated at various temperatures: (a) 90 ℃; (b) 350 ℃; (c) 400 ℃; (d) 500 ℃

Fig.3 Nitrogen adsorption-desorption isotherms for Ni-B alloys heat-treated at various temperatures(Inlet presents isotherms with relative pressure from 0.58 to 0.92)
500 ℃, namely, lose most of the activity centers. Therefore, it shows inferior catalytic activities to the former catalysts treated below 500 ℃, as shown in Fig.5. According to KIM et al[7], metal Ni with high surface area can do good to hydrolysis of NaBH4, therefore, Ni-B alloys treated at 350 ℃ and 400 ℃, which have high surface metal Ni, can still show good hydrogen generation performances. However, the 500 ℃ treated catalyst has much smaller BET surface areas than other Ni-B alloys, which leads to inferior contacts of the active sites with NaBH4, and also results in worse H2 generation performances.
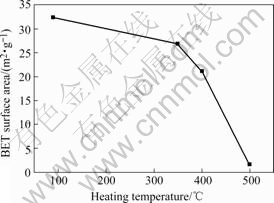
Fig.4 Evolutions of BET surface areas of Ni-B alloys heat- treated at various temperatures
Fig.6 shows the relationship between the hydrogen generation rate and reaction time, where the Ni-B alloy treated at 90 ℃ achieves a maximum hydrogen generation rate of 15.4 L/(min?g), and an average hydrogen generation rate of 13.6 L/(min?g), which can give successive hydrogen supply to a 2.2 kW PEMFC at a hydrogen utilization of 100%. Additionally, since Ni-B alloy loses the active center, atom Ni, at elevated temperatures, the hydrogen generation performance of Ni-B alloy synthesized at 500 ℃ gives inferior catalytic activity, an average hydrogen generation rate of 0.72 L/(min?g), which is just originated from metal Ni with low surface area.
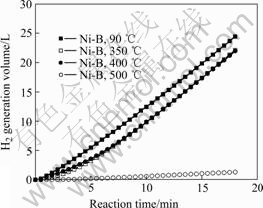
Fig.5 Relationship between hydrogen generation volume and reaction time
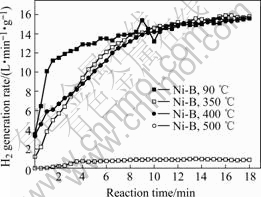
Fig.6 Relationship between hydrogen generation rate and reaction time
4 Conclusions
1) Ni-B alloys are prepared using chemical reduction method and heat-treatment at various temperatures. Hydrogen generation from sodium borohydride solution with metal boride catalysts proves it is a promising on-board hydrogen supply technique for PEMFC application.
2) The Ni-B alloy treated at 90oC achieves a maximum hydrogen generation rate of 15.4 L/(min?g), and an average hydrogen generation rate of 13.6 L/min, which can give successive hydrogen supply to a 2.2kW PEMFC at a hydrogen utilization of 100%.
3) Heat-treating results in structural evolutions of Ni-B alloys, and leads to different hydrogen generation performances. XRD patterns show that the Ni-B alloys have a tendency to decompose at elevated temperatures, and finally lost their active centers to form metal Ni, which weakens the catalytic activities. BET analysis indicates that the crystallization of Ni-B alloys is very violent between 400 ℃ and 500 ℃, which leads to a sharply decease of BET surface area, and results in inferior contacts of the active sites with NaBH4.
References
[1] WU Chuan, ZHANG H M, YI B L. Recent advances in hydrogen generation with chemical methods[J]. Prog Chem, 2005, 17(3): 423-429. (in Chinese)
[2] SCHLESINGER H I, BROWN H C, FINHOLT A E, GILBREATH J R, HOEKSTRA H R, HYDE E K. Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen[J]. J Am Chem Soc, 1953, 75(1): 215-219.
[3] BROWN H C, BROWN C A. New, highly active metal catalysts for the hydrolysis of borohydride[J]. J Am Chem Soc, 1962, 84(8): 1493-1494.
[4] AMENDOLA S C, SHARP-GOLDMANS L, JANJUA M S, SPENCER N C, Kelly M T, PETILLO P J, BINDER M. A safe, portable, hydrogen gas generator using aqueous borohydride solution and Ru catalyst[J]. Inter J Hydro Ener, 2000, 25(10): 969-975.
[5] WU C, ZHANG H M, YI B L. Hydrogen generation from catalytic hydrolysis of sodium borohydride for proton exchange membrane fuel cells[J]. Catal Today, 2004, 93/95: 477-483.
[6] BAI Y, WU C, WU F, YI B L. Carbon-supported platinum catalysts for on-site hydrogen generation from NaBH4 solution[J]. Mater Lett, 2006, 60(17/18): 2236-2239.
[7] KIM J H, LEE H, HAN S C, KIM H S, SONG M S, LEE J Y. Production of hydrogen from sodium borohydride in alkaline solution: Development of catalyst with high performance[J]. Inter J Hydro Ener, 2004, 29(3): 263-267.
[8] KOJIMA Y, SUZUKI K, FUKUMOTO K, SASAKI M, YAMAMOTO T, KAWAI Y, HAYASHI H. Hydrogen generation using sodium borohydride solution and metal catalyst coated on metal oxide[J]. Inter J Hydro Ener, 2002, 27(10): 1029-1034.
[9] KIM J H, KIM K T, KANG Y M, KIM H S, SONG M S, LEE Y J, LEE P S, LEE J Y. Study on degradation of filamentary Ni catalyst on hydrolysis of sodium borohydride[J]. J Alloys Comp, 2004, 379(1/2): 222-227.
[10] YE W, ZHANG H M, XU D Y, MA L, YI B L. Hydrogen generation utilizing alkaline sodium borohydride solution and supported cobalt catalyst[J]. J Power Sources, 2007, 164(2): 544-548.
[11] WU C, WU F, BAIY, Yi B L, ZHANG H M. Cobalt boride catalysts for hydrogen generation from alkaline NaBH4 solution[J]. Mater Lett, 2005, 59(14/15): 1748-1751.
[12] PATEL N, GUELLA G, KALE A, MIOTELLO A, PATTON B, ZANCHETTA C, MIRENGHI L, ROTOLO P. Thin films of Co-B prepared by pulsed laser deposition as efficient catalysts in hydrogen producing reactions[J]. Appl Catal A: Gen, 2007, 323(1/2): 18-24.
[13] JEONG S U, KIM R K, CHO E A, KIM H J, NAM S W, OH I H, HONG S A, KIM S H. A study on hydrogen generation from NaBH4 solution using the high-performance Co-B catalyst[J]. J Power Sources, 2005, 144(1): 129-134.
[14] DONG H, YANG H X, AI X P, CHA C S. Hydrogen production from catalytic hydrolysis of sodium borohydride solution using nickel boride catalyst[J]. Inter J Hydro Ener, 2003, 28: 1095-1100.
[15] CHLESINGER H I. Preparation of alkali metal compounds: US, 2461661[P]. 1945-01-09.
(Edited by CHEN Can-hua)
Foundation item: Project (2002CB211800) supported by the National Basic Research Program of China; project (000Y05-21) supported by the Excellent Young Scholar Research Fund of Beijing Institute of Technology; project (20060542012) supported by the Teaching & Research Fund of Beijing Institute of Technology; project(20071D1600300396) supported by the Beijing Excellent Talent Support Program
Corresponding author: WU Chuan; Tel: +86-10-68912657; E-mail: chuanwu@bit.edu.cn