J. Cent. South Univ. Technol. (2008) 15: 378-381
DOI: 10.1007/s11771-008-0071-2

Flotation of kaolinite and diaspore with
hexadecyl dimethyl benzyl ammonium chloride
HU Yue-hua(胡岳华), OUYANG Kui(欧阳魁), CAO Xue-feng(曹学锋), ZHANG Li-min(张丽敏)
(School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: Tertiary amine was synthesized from fatty amine and formaldehyde. And then the synthesized tertiary amine was used to react with benzyl chloride to synthesize hexadecyl dimethyl benzyl ammonium chloride (1627) at ambient pressure. Using the synthesized 1627 as collector, the flotation properties of diaspore and kaolinite were investigated by single mineral and mixed mineral test. The flotation mechanism of diaspore, kaolinite and 1627 was discussed based on FTIR spectra. The results show that the mass ratio of aluminum to silicate achieves 15.02 and the recovery of alumina in concentrate is 43.07% using 1627 as a collector. The 1627 is found to be a more effective and a promising collector for reverse flotation to remove aluminum-silicate minerals from bauxite.
Key words: kaolinite; diaspore; hexadecyl dimethyl benzyl ammonium chloride; dodecyl amine; reverse floatation; infra-red spectrum
1 Introduction
There are abundant bauxite resources in China, most of which are diasporic bauxite, with high mass ratio of alumina to silica (almost in the range of 4-6)[1-3]. Aluminum-silicate minerals are major gangue minerals in bauxite[4], generally in the form of kaolinite, illite, pyrophillite, feldspar and so on. The floatability of pyrophillite is better than that of kaolinite. It is therefore crucial to strengthen the collecting power for kaolinite[5-6]. In alumina industry of our country, sintering process and united process[7] are mainly used to produce alumina. However, the major disadvantages of the two methods are a large productive energy consumption, a long technological process and a high construction investment, when compared with Bayer process. The Bayer process requires mass ratio of aluminum to silicate(A/S) in raw material beyond 10. It is greatly desirable to increase A/S ratio in bauxite to fit the Bayer process. Depressing aluminum-silicate minerals and directly floating diaspore have been proved to be an efficient method to desilicate from bauxite with anionic collector[8]. But this process has drawbacks, such as a large consumption of collector, difficult to dehydrate concentrate and to remove collector from the concentrate for avoiding its unpredictable effect in the subsequent process. For these reasons, the reverse flotation process to float aluminosilicate minerals has been paid attention[9].
Dodecyl amine(DDA) is usually used in reverse flotation of iron ores, quartz and fluorite ores. However, it is found that the dodecyl amine exhibits a weak collecting power and selective ability[10-11]. So it is greatly important to explore a new type of collector. In this work, the flotation characteristics and mechanism of diaspore and kaolinite were mainly discussed with hexadecyl dimethyl benzyl ammonium chloride (1627) as collector.
2 Experimental
2.1 Synthesis of hexadecyl dimethyl benzyl ammonium chloride
Collector 1627 was first synthesized from fatty amine and formaldehyde[12].
In general, there are two ways to achieve alkylation: direct alkylation and deoxidation alkylation[13]. The latter is better. The synthesis route was to use fatty amine and formaldehyde as raw material and formic acid as reductant[12]. The adding sequence of reagent was in the order of fatty amine, formic acid, formaldehyde. The reaction is as follows:
CH3(CH2)15NH2+2HCHO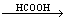
CH3(CH2)15N(CH3)2+CO2 (1)
Then the obtained tertiary amine and benzyl chloride were mixed according to the mass ratio of 1.57? 1.00[14] and reacted at 80 ℃ for 4 h. The reaction is[15]:
CH3(CH2)15N(CH3)2+C6H5CH2Cl→
CH3(CH2)15(CH3)2N+CH2C6H5Cl- (2)
The final product is yellow liquid, and the yield of this synthetic method reaches 90%.
2.2 Mineral samples
Lumps of diaspore and kaolinite, taken from Xiaoyi, Shanxi Province, were crushed, handpicked and ground in a porcelain mill. The material size is less than 74 μm. The sample purity is above 90%. The artificial mixed minerals in the test were composed of kaolinite and illite in the terms of A/S ratio of 5. The chemical compositions of mineral samples are listed in Table 1.
2.3 Flotation
Flotation tests were conducted in a 40 mL mechanical flotation cell. The impeller speed was fixed at 1 650 r/min. In each test, 3.0 g mineral sample was dispersed in 30 mL distilled water. After adjusting pH of suspension to a desired value, collector was added, and the resultant suspension was conditioned for 4 min. The flotation time was fixed as 4 min. The floated and unfloated fractions were collected and dried separately. The mass of solids in each fraction was determined accurately and used to calculate the recovery.
2.4 Infrared spectrum
The FT-IR spectra were obtained with PE system 2002 FTIR-740 (Nicolet Corporation, USA) to characterize the nature of the interaction between collector and minerals. The diffuse reflectance infrared (DRI) spectra were measured on air-dried powders whose size less than 5 μm after dealing with collector.
3 Results and discussion
3.1 Flotation of kaolinite and diaspore
The flotation recoveries of kaolinite using DDA and 1627 as collectors as a function of pH are given in Fig.1. From Fig.1, it can be seen that the recovery of kaolinite decreases with increasing the pulp pH using 1627 or DDA as collector. The collecting ability of 1627 for kaolinite is better than that of DDA.
The flotation recoveries of diaspore using DDA and 1627 as collectors as a function of pH are shown in Fig.2. It can be seen that diaspore has a better floatability in the pH range of 8-10. Obviously, the collecting ability of 1627 for diaspore is weaker than DDA. The flotation recovery is about 80% using DDA as a collector and 50% using 1627 as a collector.
The flotation responses of kaolinite and diaspore as a function of reagent dosage using DDA and 1627 as collectors are shown in Fig.3 and Fig.4, respectively. With the increase of collector dosage, the recoveries of kaolinite and diaspore increase. Collector 1627 exhibits stronger collecting power for kaolinite in the pH range of

Fig.1 Flotation recoveries of kaolinite as function of pH using 1627 and DDA as collectors

Fig.2 Flotation recoveries of diaspore as function of pH using 1627 and DDA as collectors
Table 1 Chemical compositions of mineral samples (mass fraction, %)


Fig.3 Relationship between recovery of kaolinite and reagent dosage (pH=5.0-5.5)
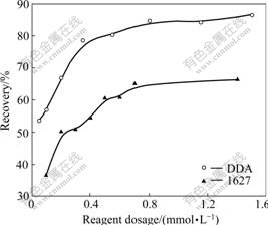
Fig.4 Relationship between recovery of diaspore and reagent dosage (pH=5.0-5.5)
5.0-5.5. But the collecting ability of 1627 for diaspore is weaker than that of DDA. It suggests that 1627 is a more effective collector for reverse flotation of aluminosilicate minerals from diaspore.
3.2 Separation of artificial mixed minerals
The reverse flotation separation of artificial mixture was carried out by using 0.2 mmol/L 1627 as collector, and adjusting pH of pulp to 5.0-5.5. Table 2 lists the results of the separation test.
From Table 2, it can be seen that the mass ratio of Al2O3 to SiO2 in the feed is 5.00 and that in concentrate with 43.07% in recovery of alumina is 15.02. The result indicates that the collector 1627 has a better selectivity, but its collecting power is not strong enough.
4 Infrared spectrum analysis
4.1 Infrared spectrum analysis of 1627 adsorption on diaspore
Infrared spectra of 1627, diaspore, and diaspore conditioned with 0.2 mmol/L 1627 are presented in Fig.5. There is O—H stretching at 2 921-3 007 cm-1 in the spectrum of diaspore. The peaks at 2 119 and 1 985 cm-1 are due to vibration of O—H. The peaks at 1 082 and 973 cm-1 are due to bending vibration of O—H. The peak at 752 cm-1 is due to stretching of Al—O. The distinct peaks of collector 1627 at 2 919 and 2 850 cm-1 are due to stretching of —CH3 and —CH2, respectively. The peak around 1 300 cm-1 is due to stretching of C—N[16]. The peaks in the range of 1 400-1 600 cm-1 are due to —CH3 and —CH2 deformation. The peaks in the ranges of 1 006-1 203 cm-1 and 698-730 cm-1 are vibrating modes of —CH3 and —CH2 on the surface, respectively. After adding 1627, new stretching of C—N appears at 1 344 cm-1, deformation vibration of —CH3 and —CH2 appears at 1 469 and 1 592 cm-1, respectively. The evidences mentioned above demonstrate that 1627 is adsorbed by diaspore. The characteristic peaks of diaspore at 2 921, 2 119 and 752 cm-1 do not shift, indicating that the adsorption of 1627 on diaspore is dominated by very weak physical adsorption.
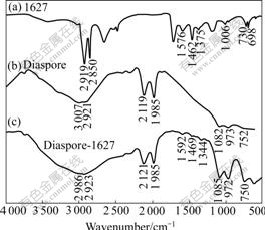
Fig.5 FTIR spectra of 1627 (a), diaspore (b) and diaspore-1627 (c)
Table 2 Test results of flotation of artificial mixed minerals using 1627 as collector
4.2 Infrared spectrum analysis of 1627 adsorption on kaolinite
Infrared spectra of collector 1627, kaolinite, and kaolinite in the presence of 0.2 mmol/L 1627 are shown in Fig.6. It is clear that the IR of kaolinite-1627 is different from that of kaolinite. The distinct peaks appeared at 1 576 and 1 462 cm-1 are due to vibration of aromatic ring in the infrared spectrum of 1627.

Fig.6 FTIR spectra of 1627 (a), kaolinite (b) and kaolinite-1627 (c)
In the infrared spectrum of kaolinite treated with 1627, new peaks appear at 2 922 and 2 852 cm-1, which are contributed to the stretching vibration of —CH3 and —CH2. And the characteristic peaks of 1627 are transferred to 1 596 and 1 468 cm-1, indicating that the adsorption of 1627 on kaolinite is dominated by strong physical adsorption.
4.3 Effect of structure of 1627 on flotation property
There is a benzyl on atom N of 1627, and the electron-pair on atom N of amidogen can conjugate benzene ring, but electron-pair on alkyl of DDA cannot. Therefore, it can be speculated that the offering electronic effect of benzyl is stronger than that of alkyl. The electronegativity of 1627 is 5.05. Consequently, the ability of 1627 combined with kaolinite through electrostatic force is better than that of DDA.
In sum, the collecting ability and the selectivity of cationic collector 1627 for kaolinite are better than those of DDA, and the pH range of flotation is wider.
5 Conclusions
1) Hexadecyl dimethyl benzyl ammonium chloride is synthesized by the means of deoxidation alkylation at an ambient pressure, using fatty amine and formaldehyde as raw material and formic acid as reductant. The yield of this synthetic method reaches 90%.
2) The mass ratio of aluminum to silicate achieves 15.02 and the recovery of alumina in concentrate is 43.07% using 1627 as collector.
3) The collecting ability of 1627 for kaolinite is stronger than that of DDA, while the collecting ability of 1627 for diaspore is weaker than that of DDA. Therefore, 1627 is a suitable collector for bauxite reverse flotation.
4) It should focus on improving the process technology in order to not only increase the mass ratio of aluminum to silicate but also improve the alumina recovery in concentration.
References
[1] ZHAO Zu-de. Bauxite and alumina industry of world [M]. Beijing: Science Process, 1994. (in Chinese)
[2] GUO Jian. A study on separation diaspore and kaolinite by reverse flotation [D]. Beijing: University of Science and Technology Beijing, 2001. (in Chinese)
[3] QIN Wen-qing, HU Yue-hua, QIU Guan-zhou, JIANG Hao. Interaction and flotation of diaspore with alkylamine hydrochlorides [J]. Trans Nonferrous Met Soc China, 2001, 11(3): 430-433.
[4] HU Yue-hua, CAO Xue-feng, LI Hai-pu, JlANG Yu-ren, DU Ping. Synthesis of N–decyl–1, 3–diaminopropanes and its flotation properties on aluminium silicate minerals [J]. Trans Nonferrous Met Soc China, 2003, 13(2): 417-420.
[5] HU Yue-hua, JIANG Hao, QIU Guan-zhou, WANG Dian-zuo. Solution chemistry of flotation separation of diasporic bauxite [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(1): 125-130. (in Chinese)
[6] ZHAO Sheng-gui, ZHONG Hong, LIU Guang-yi. Effect of quaternary ammonium salts on flotation behavior of aluminosilicate minerals [J]. J Cent South Univ Technol, 2007, 14(4): 500-503.
[7] HU Yue-hua, WANG Yu-hua, WANG Dian-zuo. Flotation chemistry and desilication of bauxite [M]. Beijing: Science Press, 2004. (in Chinese)
[8] JIANG Hao. Studies on solution chemistry of interactions between cationic collectors and aliminosilicate aluminum minerals in bauxite flotation desilica [D]. Changsha: Central South University, 2004. (in Chinese)
[9] LIU Pi-wang, ZHANG Lun-he, ZHANG Xiao-feng, PEI Yu, LI Kai-gong. Theoretical basis of new technology in predesilicification and bauxite dressing-buyer process and industry technique of new technology [J]. Journal of Chemical Industry and Engineering, 2000, 51(6): 734-739. (in Chinese)
[10] CAO Xue-feng, HU Yue-hua, XU Jing. Synthesis of γ-alkoxy-propylamines and their collecting properties on aluminosilicate minerals [J]. J Cent South Univ Technol, 2004, 11(3): 280-285.
[11] REN Jian-wei, WANG Yu-hua. Reverse flotation desilicates on iron ores [J]. China Mining Magazine, 2004, 13(4): 70-72. (in Chinese)
[12] CAO Xue-feng. Synthesis of collectors to catch aluminum silicate minerals and studies on structure-activity relationship [D]. Changsha: Central South University, 2004. (in Chinese)
[13] LI Shu-wen, FAN Ru-lin. The handbook of organic chemistry [M]. Shanghai: Shanghai Science Technology Press, 1981. (in Chinese)
[14] ZOTOV V I, MANKOVICH L. Method for producing alkyl- dimethylbenzylammonium chlorides [P]. CN03809057. 2005-07- 27.
[15] ZHU Guang-jun, ZHANG Xi-he, LIU Bin, XU Xiao-feng. Synthesis study of a new surfactant oxidated tertiary amine [J]. Fine Chemicals, 1994, 11(1): 1-3. (in Chinese)
[16] XIE Jing-xi. Application of infrared spectrum in organic chemistry and medicament [M]. Beijing: Science Press, 1987. (in Chinese)
(Edited by YANG Hua)
Foundation item: Project(2006AA06Z120) supported by the National High-Tech Research and Development Program of China; Project(2005CB623701) supported by the Major State Basic Research Development Program of China
Received date: 2007-11-26; Accepted date: 2008-01-29
Corresponding author: HU Yue-hua, Professor; Tel: +86-731-8879815; E-mail: HYH@mail.csu.edu.cn