J. Cent. South Univ. (2012) 19: 2340-2347
DOI: 10.1007/s11771-012-1280-2
Railway tunnel concrete lining damaged by formation of gypsum, thaumasite and sulfate crystallization products in southwest of China
MA Kun-lin(马昆林)1, 2, 3, LONG Guang-cheng(龙广成)1, XIE You-jun(谢友均)1
1. School of Civil Engineering, Central South University, Changsha 410075, China;
2. National Engineering Laboratory for Construction Technology of High Speed Railway, Changsha 410075, China;
3. Department of Mechanical Engineering, Hunan Industry Polytechnic, Changsha 410208, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: The railway tunnel concrete lining exposed to sulfate-bearing groundwater beyond 40 years in southwest of China was investigated. Field investigation, sulfate ions content and corroded products analysis, macro/microscopic test were carried out. Results show that under the tunnel concrete lining structure and its served environmental conditions, sulfate solutions permeate concrete lining and accumulate on windward-side of concrete lining, resulting in the increase of sulfate ions content on windward-side and the diffusion of sulfate ions from windward-side to waterward-side, which cause the concrete lining of windward-side damaged seriously but the waterward-side of concrete lining is still in perfect condition. It is confirmed that structural characteristic of tunnel and environmental conditions lead to physical attack with the leaching of concrete and sodium sulfate crystallization as well as chemical corrosion with formation of gypsum in high sulfate concentration and formation of thaumasite in proper temperature rather than formation of ettringite. These achievements can provide academic and technical supports for understanding the deterioration mechanism of concrete lining as well as constructing railway tunnel under sulfate attack.
Key words: railway tunnel; concrete lining; sulfate attack; deterioration mechanism
1 Introduction
Generally, concrete materials are characterized by durability qualities. However, under certain circumstances, the interaction with water containing soluble salts may lead to serious chemical alterations of concrete which shorten the service life of constructions. Because of the unusual structural form, in which one side contacts with ground water, called waterwind-side, and the other side contacts with air, called windward-side, tunnel concrete lining is easy to be attacked by ground water containing sulfate, and the sulfate attack on concrete lining can result in serious damage of tunnel structure [1-3]. In the case of tunnels and other underground constructions, the costs for renovations are extremely high. Therefore, in order to initiate protective countermeasures against corrosive damages, it is important to understand the deterioration mechanism of concrete lining under groundwater attack.
Cement-based materials exposed to sulfate-bearing solutions such as some natural or polluted ground waters (external sulfate attack), or under the action of sulfates present in the original mix (internal sulfate attack) can show signs of deterioration [4-5]. Sulfate attack on concrete has been seemed as a series of complex problems [6], but it is generally accepted that there are two types of sulfate attack on cement-based materials, namely, chemical corrosion and physical attack. Chemical corrosion is that sulfate ions react with ionic species of the pore solution and hydrated products of cement to form ettringite ([Ca3Al (OH)6·12H2O]2·(SO4)3·
2H2O), gypsum (CaSO4·2H2O) or thaumasite (Ca3[Si(OH)6·12H2O]·(CO3)·SO4) or mixtures of these phases [7-9]. Generally, gypsum is the primary product of sulfate attack at high concentrations of sulfate ions, and when the pH of the pore water in hydrated cement paste falls below about 11.5, ettringite is not stable and decomposes to gypsum. Ettringite is the product of sulfate attack at low concentrations of the sulfate ions, and the formation of thaumasite needs such environmental condition that sulfate and carbonate are existent synchronously, and environmental temperature is low (<15 ℃) [10]. The precipitation of these solid phases can lead to strain within the cement-based materials, inducing expansion, strength loss, spalling and severe degradation. Physical attack means the reversible phase change of sulfate in concrete pores under the influence of environment and other conditions [11-12]. For example, when temperature and relative humidity change, the phase change between anhydrous sodium sulfate (thenardite) and decahydrate (mirabilite) will take place [13-14]. If physical crystallization attack takes place in pores at or near the surface of concrete, large pressure may develop with consequent deleterious action. However, under a certain environment, the formations of corroded products of concrete attacked by sulfate are different [15].
In this work, the underlying deterioration mechanisms of tunnel concrete lining exposed to sulfate environment for long time was investigated. Based on the results of field investigations, detailed analysis of drilled concrete samples from concrete lining in lab, combining the served environment as well as structural form of tunnel, a succession of corrosive reactions and deterioration process can be put forward. The corrosion reactions include ion migration, salts precipitation/ reprecipitation and formation of new products. It is useful to further understand the deterioration mechanism of concrete under tunnel environment.
2 Experimental
2.1 Field investigation
According to the requirement of railway authorities, several badly corroded tunnels in Cheng-Kun railway line were investigated in details. The items of field investigation included damage characteristic of concrete lining, served environmental conditions of tunnel concrete lining, the chemical composition of the ground water, the carbonation depth of concrete lining of windward-side as well as served age of concrete lining.
2.2 Laboratory investigations of field concrete
According to the results of field investigation, concrete samples with dimension of d50 mm×300 mm drilled vertically from concrete lining surface were cored carefully in locations showing representative deterioration phenomena. After cutting and drying, fractions of the material were chosen for microscopic analytical investigation. The barium sulfate gravimetric method was used to test the  content in different concrete layers from windward-side to waterward-side. The main processes included drilling the core sample from the specimen, cutting the core sample into slices, pulverizing the slices into powder layer by layer, accurately weighting the powder samples and determining the sulfate ion contents of every layer samples. The microstructure of concrete was observed by methods of scanning electron microscope (SEM) and energy disperse spectroscopy (EDS). Corrosion products and suspected substance in concrete samples were analyzed by method of X-ray diffraction (XRD). The chemical composition of ground was tested by the method of chemical titration. Carbonation of concrete lining was tested by the method of spraying phenolphthalein absolute alcohol solution.
content in different concrete layers from windward-side to waterward-side. The main processes included drilling the core sample from the specimen, cutting the core sample into slices, pulverizing the slices into powder layer by layer, accurately weighting the powder samples and determining the sulfate ion contents of every layer samples. The microstructure of concrete was observed by methods of scanning electron microscope (SEM) and energy disperse spectroscopy (EDS). Corrosion products and suspected substance in concrete samples were analyzed by method of X-ray diffraction (XRD). The chemical composition of ground was tested by the method of chemical titration. Carbonation of concrete lining was tested by the method of spraying phenolphthalein absolute alcohol solution.
3 Results and discussion
3.1 Field investigation
Figure 1 show the appearances of concrete lining of tunnel attacked by ground water. Figure 1(a) shows the panorama of concrete lining attacked by ground water. As can been seen from Fig. 1(a), on the concrete lining surface from bottom in sidewall to vault, lots of white substances are on concrete surface. Figure 1(b) shows the leaking of ground water from concrete lining of windward-side surface. Figure 1(c) shows the white crystallization products on concrete lining surface. According to field investigation, it is found that the representative deterioration characteristics of tunnel lining concrete are as follows. In some spots, ground water permeates the concrete lining and reaches windward-side. Ground water on concrete lining surface is evaporated, and lots of white substances are separated with the effect of evaporation. Some zones are leached by ground water seriously, and the aggregates are naked. It is found that the concrete lining of windward-side is damaged seriously but the waterward-side of concrete lining is still in perfect condition.

Fig. 1 Photos of tunnel concrete lining attacked by groundwater: (a) Concrete lining of windward-side; (b) Concrete lining surface; (c) Crystallization products on concrete surface
It is also found that it is windy in tunnels especially in the entrance of tunnels, and the evaporation of water on concrete surface is quick. Negative pressure resulted from trains getting across tunnel with high speed accelerates the migration of ions in concrete lining. When groundwater permeates concrete lining and get to windward-side, water is evaporated fast and salts are precipitated from solution.
According to related information from railway authorities, most of these tunnels in Cheng-Kun railway line finished in 1968 and all of them have served beyond 40 years. The carbonation depths of concrete lining of windward-side are beyond 40 mm and the environmental temperature in tunnel keeps almost constant (about 20- 25 ℃) according to spot test.
Table 1 presents the tested results of chemical compositions of ground water at five different sampling spots in tunnel. As can be seen from Table 1, the dominating anion in the groundwater is  with minor amounts of
with minor amounts of 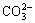 and Cl-, and the average sulfate ions concentration is about 1 700 mg/L with a maximal concentration of 2 031 mg/L and minimal concentration of 1 592 mg/L.
and Cl-, and the average sulfate ions concentration is about 1 700 mg/L with a maximal concentration of 2 031 mg/L and minimal concentration of 1 592 mg/L.
3.2 Leaching of concrete
Chemical compositions of groundwater are quite different from a representative concrete pore solution, which leads to effect of medium transfer between the ground water and the saturated cement paste. Percolating ground water along pathways through concrete efficiently leaches alkalis and portlandite, leading to a “leaching zone” characterized by the reduction of portlandite while calcium silicate hydrate (C-S-H) phases still present. During further leaching, all portlandite is removed and the pH reduces rapidly. C-S-H phases are not stable anymore and start to dissolve [16], leading to a “corrosion zone”. During these leaching processes, the density of the hardened cement paste is reduced accompanied by micro-cracking and therefore the permeability properties increase.
As can be seen from Fig. 2(a), the hardened cement paste on concrete lining surface of windward-side is leached by ground water, resulting in the exposing of the underlying aggregate face. The formation of leaching zone is related to the dissolving of portlandite compared to unaltered paste. Figure 2(b) gives the SEM image of leaching zone. As can been seen from Fig.2 (b), the cement paste in leaching zone is incompact and porous, and some aggregates can be seen in cement paste. From the analysis, it is confirmed that the leaching of concrete brings about the weak zones, which accelerates the deterioration of concrete.
Table 1 Chemical composition and pH value of ground water


Fig. 2 Leaching of concrete lining: (a) Macro-photo of leaching zone; (b) SEM image of leaching zone
3.3 Deleterious ions distribution profile
According to chemical composition analysis of groundwater, deleterious ions such as  and Cl- were tested in concrete samples. Figure 3 shows the tested results of content of
and Cl- were tested in concrete samples. Figure 3 shows the tested results of content of  and Cl- in concrete samples. As can be seen from Fig. 3(a), content of
and Cl- in concrete samples. As can be seen from Fig. 3(a), content of  is the largest in concrete lining of windward-side, and it decreases gradually from concrete lining of windward-side to inner. When the distance from concrete lining outside surface is beyond 200 mm, the content of
is the largest in concrete lining of windward-side, and it decreases gradually from concrete lining of windward-side to inner. When the distance from concrete lining outside surface is beyond 200 mm, the content of  almost keeps constant. Obviously, the change of content of
almost keeps constant. Obviously, the change of content of  in concrete lining comes from the ingression of sulfate. After sulfate permeates concrete lining and transfers from the inner side which contacts with ground water to outside which contacts with air, the sulfate accumulates on concrete lining surface contacting with air. Under the special tunnel environment, such as wind, low relative humidity and negative pressure, ground water on windward-side is evaporated quickly, and the solution concentration on concrete lining surface increases, which results in diffusion effect of solution from high concentration zone to low concentration zone. According to Fig. 3(b), the content of Cl- in concrete lining is almost constant, which can be explained by the fact that the Cl- in concrete lining is mainly from raw materials but not from ground water. The content of chloride ions in ground water is tiny according to Table 1. Therefore, sulfate attack is the dominating reason resulting in concrete lining deterioration.
in concrete lining comes from the ingression of sulfate. After sulfate permeates concrete lining and transfers from the inner side which contacts with ground water to outside which contacts with air, the sulfate accumulates on concrete lining surface contacting with air. Under the special tunnel environment, such as wind, low relative humidity and negative pressure, ground water on windward-side is evaporated quickly, and the solution concentration on concrete lining surface increases, which results in diffusion effect of solution from high concentration zone to low concentration zone. According to Fig. 3(b), the content of Cl- in concrete lining is almost constant, which can be explained by the fact that the Cl- in concrete lining is mainly from raw materials but not from ground water. The content of chloride ions in ground water is tiny according to Table 1. Therefore, sulfate attack is the dominating reason resulting in concrete lining deterioration.
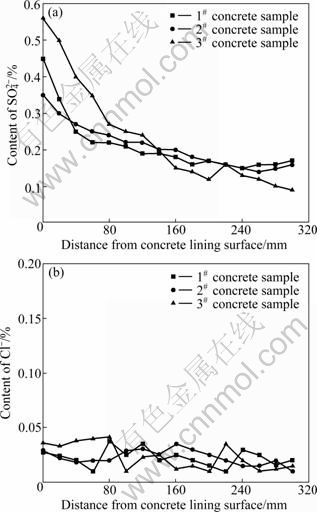
Fig. 3 Deleterious ions profile in concrete lining samples: (a) Content of 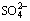 in concrete samples; (b) Content of Cl- in concrete samples
in concrete samples; (b) Content of Cl- in concrete samples
Based on the analysis above, it can be concluded that the high concentration of sulfate ions at surface layer of concrete lining brings about the serious damage of windward-side of concrete lining.
3.4 Formation of chemical corrosion products
Figure 4 shows the analysis of corroded products from windward-side of concrete lining under the attack of ground water. As can been seen from SEM image in Fig. 4(a), there are a large number of columnar crystallization products in concrete lining, and these columnar crystallization products contain mainly elements of calcium and sulphate according to the EDS analysis (Fig. 4(b)). Furthermore, on the basis of XRD pattern analysis (Fig. 4(c)), the substances in concrete lining mainly contain the crystals of CaSO4·2H2O, CaCO3 and SiO2. According to the shape of crystal and the analysis, it can be inferred that plenty CaSO4·H2O crystals form on windward-side of concrete lining. Gypsum (CaSO4·2H2O) can be formed by sodium sulfate reacting with calcium hydroxide in high sulfate environment. Plenty of ground water permeates concrete lining and accumulate on windward-side. Under tunnel environment, the evaporation of ground water on windward-side is quick, resulting in the development of sulfate solution concentration. Therefore, under such environment, the condition of formation of gypsum is satisfied. According to field investigation, sodium sulfate is the predominant salt involved in groundwater in southwest of China. The formation of gypsum in concrete may lead to the increase of stress. The calcium carbonate (CaCO3) and quartz (SiO2) may come from carbonation of concrete and raw materials, respectively.
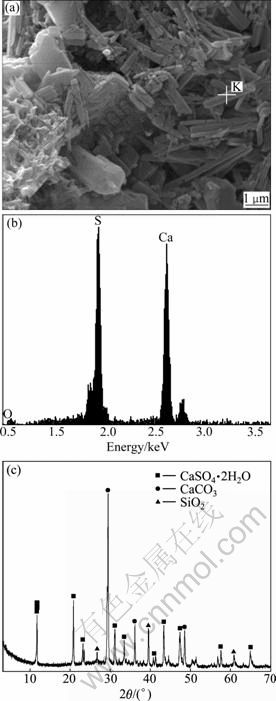
Fig. 4 Corroded products analysis of concrete lining: (a) SEM image of concrete lining sample A; (b) EDS result of spot K in Fig. 4(a); (c) XRD pattern of sample A in concrete lining
Figure 5 shows that the corroded products are on the concrete lining of the windward-side under the attack of sulfate. As can be seen from the SEM image in Fig. 5(a), there are many tiny acicular crystals among hydrated products. And these tiny acicular crystals mainly contain such elements as calcium, silicon and sulfate according to the EDS analysis (Fig. 5(b)). Furthermore, on the basis of XRD pattern analysis (Fig. 5(c)), the substances in concrete lining mainly contain thaumasite (Ca3[Si(OH)6·
12H2O]·(CO3)·SO4), and gypsum (CaSO4·H2O) ettringite ([Ca3Al(OH)6·12H2O]2·(SO4)3·2H2O). Thaumasite and ettringite are very similar in molecular structure, and their XRD patterns are almost the same [10]. In this work, it is hard to find ettringite in concrete, but thaumasite is the common substance in concrete lining surface according to analysis of corroded products.

Fig. 5 Corroded products analysis of concrete lining: (a) SEM image of concrete lining sample B; (b) EDS result of spot M in Fig. 5(a); (c) XRD pattern of sample B in concrete lining
Based on the analysis of corroded products, it can be inferred that the formation of thaumasite is one of the reasons causing concrete lining deterioration. The existence of sulfate, carbonate and low temperature (<15 ℃) are the necessary conditions to form thaumasite in literatures. In the investigated tunnels, environmental temperature is kept between 20 and 25 ℃, but plenty of thaumasites are found in concrete lining, showing that low temperature may not a prerequisite for the formation of thaumasite. In certain conditions, thaumasite is formed during long time sulfate attack with the existence of carbonate in almost fixed temperature.
3.5 Formation of physical crystallization products
Figure 6 also shows the corroded products from concrete lining on windward-side under the attack of ground water. As can be seen from SEM image in Fig. 6(a), concrete is incompact and lots of crystallization products exist. And these crystals mainly contain such elements as sodium and sulfate according to the EDS analysis (Fig. 6(b)). Furthermore, on the basis of XRD pattern analysis (Fig. 6(c)), the substances in concrete lining mainly contain sodium sulfate crystals including thenardite (Na2SO4) and mirabilite (Na2SO4·10H2O). The formation of these crystals comes from the precipitation of sodium sulfate after ground water permeates concrete lining and gets to windward- side.
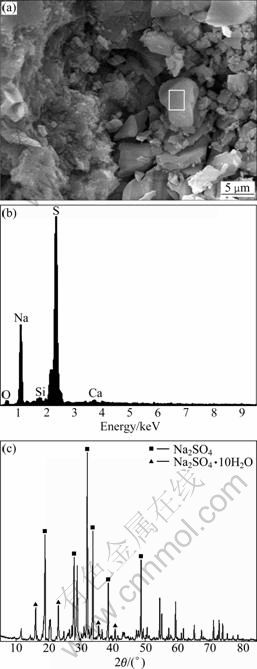
Fig. 6 Corroded products analysis of concrete lining: (a) SEM image of concrete lining sample C; (b) EDS result of spot N in Fig. 6(a); (c) XRD pattern of sample C in concrete lining
4 Discussions
Salt solution attack on concrete structure must be in definite environmental condition. In this work, railway tunnels are the structures in which one side contacts with ground water, soil or rock (called waterward-side) and the other side contacts with air (called windward-side). Because of low relative humidity, wind, negative pressure in tunnel and porosity of concrete, groundwater can permeate concrete lining to the windward-side in some zones. Water in solution on windward-side is evaporated quickly, and solution concentration rises increasingly. Therefore, windward-side concrete is damaged by chemical corrosion as well as physical crystallization attack. Chemical corrosion is caused by sulfate ions taking reaction with hydration products of cement in high concentration and then CaSO4·2H2O is the product of chemical reaction. It is found that long-time sulfate attack and concrete carbonation at certain temperature (about 20-25 ℃) can also result in the formation of thaumasite in concrete. Physical crystallization attack is caused by separation of sodium sulfate crystal from solution because of the increase of solution concentration resulted from water evaporation.
According to field investigation and experimental analysis above, the main deterioration mechanisms of tunnel concrete lining under such condition are sulfate chemical corrosion in high  concentrations and sodium sulfate physical crystallization attack, respectively. The corroded products are gypsum, thaumasite, ettringite, sodium sulfate or mixtures of these phases. The formation of thaumasite is not at low temperature. The descriptions of deterioration process of tunnel lining concrete by sulfate attack based on field investigation and test analysis results in laboratory are shown in Fig. 7. As can be seen from Fig. 7, groundwater permeates concrete lining by penetration effect, capillary effect and diffusion effect. Under the special environment condition in tunnel, such as low relative humidity, high wind speed and negative pressure (the negative pressure in tunnels caused by train getting across tunnel with high speed), groundwater on concrete lining windward-side is evaporated quickly and concentration of salt solution increases. Therefore, physical attack of salt solution and chemical corrosion of salt solution take place, resulting in the deterioration of tunnel lining concrete structure. Figure 8 shows the typical engineering condition of tunnel structure under sulfate attack.
concentrations and sodium sulfate physical crystallization attack, respectively. The corroded products are gypsum, thaumasite, ettringite, sodium sulfate or mixtures of these phases. The formation of thaumasite is not at low temperature. The descriptions of deterioration process of tunnel lining concrete by sulfate attack based on field investigation and test analysis results in laboratory are shown in Fig. 7. As can be seen from Fig. 7, groundwater permeates concrete lining by penetration effect, capillary effect and diffusion effect. Under the special environment condition in tunnel, such as low relative humidity, high wind speed and negative pressure (the negative pressure in tunnels caused by train getting across tunnel with high speed), groundwater on concrete lining windward-side is evaporated quickly and concentration of salt solution increases. Therefore, physical attack of salt solution and chemical corrosion of salt solution take place, resulting in the deterioration of tunnel lining concrete structure. Figure 8 shows the typical engineering condition of tunnel structure under sulfate attack.

Fig. 7 Deterioration process of tunnel concrete lining under sulfate attack

Fig. 8 Type of engineering of tunnel concrete lining attacked by sulfate
5 Conclusions
1) Compared with concrete lining contacting with groundwater (waterward-side), concrete lining contacting with air (windward-side) is seriously damaged by ground water. The environmental conditions of served tunnel play important roles in the corrosion damage of tunnel structure. The severity of concrete lining deterioration is mainly dependent on environmental parameters in tunnel like the low relative humidity, high wind speed and negative pressure.
2) The deterioration of concrete lining under sulfate attack is a very complex process, including physical attack and chemical corrosion. The leaching of concrete and precipitation of sodium sulfate crystals are the main physical attacks on concrete lining. Chemical corrosion of concrete lining is mainly caused by the formation of gypsum and thaumasite rather than the formation of ettringite.
3) In all investigated tunnel concrete lining samples, the content of sulfate ions is the largest on the surface of concrete lining of windward-side, and decreases from windward-side to inner of concrete lining. The processes of leaching and the formation of sulfate minerals, predominantly thaumasite and gypsum, have been detected. Many parameters control the formation of thaumasite, but low temperature (<15℃) is not a prerequisite for the formation of thaumasite. In southwest of China, in tunnel regions with thaumasite- related concrete, the temperature is always between 20 and 25 ℃.
References
[1] LEEMANN A, LOSER R. Analysis of concrete in a vertical ventilation shaft exposed to sulfate-containing groundwater for 45 years [J]. Cement and Concrete Composites, 2011, 33(1): 74-83.
[2] ROMER M, HOLZER L, PFIFFNER M. Swiss tunnel structures: Concrete damage by formation of thaumasite [J]. Cement and Concrete Composites, 2003, 25(8): 1111-1117.
[3] LONG Guang-cheng, XIE You-jun, DENG De-hua, LI Xiao-kun. Deterioration of concrete in railway tunnel suffering from sulfate attack [J]. Journal of Central South University of Technology, 2011, 18(3): 881-888.
[4] FRANK R, RAOUL J. The deterioration of mortar in soleplates environments [J]. Construction and Building Materials, 1999, 13(2): 321-327.
[5] FREDRIK P G, JACQUES M, ERIC S. Durability of concrete- Degradation phenomena involving detrimental chemical reactions [J]. Cement and Concrete Research, 2008, 38(2): 226-246.
[6] NEVILL E A. The confused world of sulfate attack on concrete [J]. Cement and Concrete Research, 2004, 34(8): 1275-1296.
[7] LEE S T, HOOTON R D, JUNG H S, PARK D H. Effect of limestone filler on the deterioration of mortars and pastes exposed to sulfate solutions at ambient temperature [J]. Cement and Concrete Research, 2008, 38(1): 68-76.
[8] NORAH C. The occurrence of thaumasite in modern construction- A review [J]. Cement and Concrete Composition, 2002, 24(4): 393-402.
[9] NEIL L. A review of the experience of thaumasite sulfate attack by the UK Highways Agency [J]. Cement and Concrete Composites, 2003, 25(8): 1051-1058.
[10] GAO Li-xiong, YAO Yan, WANG Ling. Effects of temperatures on thaumasite formation [J]. Journal of the Chinese Ceramic Society, 2005, 33(4): 525-528.
[11] SCHERER G W. Crystallization in pores [J]. Cement and Concrete Research, 1999, 29(9): 1347-1358.
[12] ESPINOSA R M, FRANKE L, DECKELMANN G. Model for the mechanical stress due to the salt crystallization in porous materials [J]. Construction and Building Materials, 2008, 22(9): 1350-1367.
[13] NICHOLAS T, FLATT R J, SCHERER G W. Crystallization damage by sodium sulfate [J]. Journal of Cultural Heritage, 2003, 4(2): 109-115.
[14] MA Kun-lin, XIE You-jun, LONG Guang-cheng. Deterioration behaviors of sulfate crystallization attack on cement-based material [J]. Journal of Central South University: Science and Technology, 2010, 41(2): 303-310. (in Chinese)
[15] MA Kun-lin, XIE You-jun, LONG Guang-cheng. Characteristics of cement mortar by physical attack of sodium sulfate [J]. Journal of the Chinese Ceramic Society, 2007, 35(10): 1376-1382.
[16] ANDAC M, GLASSER F P. Long-term leaching mechanisms of Portland cement-stabilized municipal solid waste fly ash in carbonated water [J]. Cement and Concrete Research, 1999, 29(1): 179-186.
(Edited by HE Yun-bin)
Foundation item: Project(51108463) supported by the National Natural Science Foundation of China; Project(11B041) supported by Scientific Research Fund of Hunan Provincial Education Department of China; Project(NCET-10-0839) supported by Ministry Education of China
Received date: 2011-05-31; Accepted date: 2011-09-30
Corresponding author: LONG Guang-cheng, Professor, PhD; Tel: +86-731-82656568; E-mail: scc2005@csu.edu.cn