Article ID: 1003-6326(2005)02-0375-04
Interface evolution of TiAl/Ti6242 transient liquid phase joint using Ti, Cu foils as insert metals
DUAN Hui-ping(段辉平)1, K. H. Bohm2, V. Ventzke2, 
(1. School of Materials Science and Engineering, Beijing University of Aeronautics and Astronautics, Beijing 100083, China;
2. Institute for Materials Research, GKSS Research Center Geesthacht,Geesthacht D-21502, Germany)
Abstract: The interface evolution of TiAl/Ti6242 joint produced by transient liquid phase(TLP) bonding with Ti, Cu foils as insert metals was investigated. The results show that the surface oxide layer on TiAl plays a very important role in the formation process of the joint. A ‘bridge’ effect is observed because of the presence of the oxide layer on the surface of TiAl. The diffusion behavior of Cu atoms in TiAl is strongly controlled by the vacancies beneath the surface of TiAl. Based on the interface diffusion and interface wettability, a mechanism for the effect of bonding pressure, bonding temperature, holding time and stacking sequence of the insert foils on the joint formation process were proposed.
Key words: TiAl; Ti6242 transient liquid phase bonding; intermetallics; interface evolution; mechanism CLC number:
Document code: A
1 INTRODUCTION
TiAl-base alloy is an attractive candidate material used in aerospace, automotive and power generation industries, but its poor workability makes it impossible to fabricate a complex integrity component in some cases. So it is important to employ a suitable bonding technique to join TiAl-base alloy to itself or to other materials.
Recently transient liquid phase(TLP) bonding has been gotten more and more attention to join advanced materials[1, 2]. The principle of this method has been introduced elsewhere[3]. The process and phenomenon of TLP bonding are very similar to those of the conventional brazing. The most important difference between TLP bonding and brazing is the solidification behavior of the liquid phase formed during bonding. The difference can become much clear by checking the stages which the bonding processes have undergone[4, 5].
TLP bonding can be utilized to join intermetallics to itself or to superalloys. However, most of the current investigations focus on NiAl intermetallics. Gale et al[6, 7] and Moore and Kalinowski[8] elaborately investigated the bonding process and interface structure of NiAl TLP joints. Furthermore, Strum et al[9] succeeded in producing bondline-free NiAl TLP joint using 0.165mm pure Al and 0.25mm Ni layers in diameter as insert metals by electron beam evaporation onto preheated NiAl substrates. Recently Butts et al[10] investigated TLP bonding of TiAl-alloy using a mixture composed of Cu powders and atomized TiAl-alloy powders as interlayer. Moreover the oxidation resistance of the similar joints has been estimated in the work conducted by Fergus et al[11].
The application of TLP in joining of intermetallics to metals has been investigated widely. Gale et al[12, 13] joined NiAl to Ni with Ni-Si-B insert metal respectively. Microstructural development in NiAl/Ni-Si-B/Ni joint showed that aluminum transferred from NiAl substrate to the initially Al-free joint and a martensite transformation of NiAl substrate occurred. Moreover liquidation has also been found in the joint. In order to avoid the liquation of Ni substrate, an inset foil alternative, pure copper, was chosen to join NiAl and Ni-base alloy[14]. Investigation on the interface evolution of the joint indicates that the isothermal solidification of the joint occurs primarily by epitaxial growth of β phase from the NiAl substrate into the joint. Using corresponding bulk alloys with the same composition as that of the phases detected in the NiAl/Cu/Ni joint, an analogical examination was made to clarify the interface development and mechanical property of the dissimilar material joints by Gale and Abdo[15].
As one of the microstructure-tailorable alloy, Ti6242 has potential to be designed to obtain desired comprehensive properties for given aerospace applications. In this paper, TLP bonding technique was employed to join Ti6242 and TiAl-base alloys with Ti and Cu foils as insert metals. The interface structure of TiAl/Ti6242 TLP joint was investigated in details. Based on the experimental results, the mechanism of the interface evolution was proposed.
2 EXPERIMENTAL
The master materials to be joined were Ti-42Al-2Cr(mass fraction, %) and Ti6242 alloys. The alloys were machined to the size of 14mm×14mm×4mm and the mating surfaces were ground with 10μm SiC abrasive paper. Ti and Cu foils with thickness of 50μm and 20μm respectively were used as insert metals. All of the raw materials were cleaned in acetone with ultrasonic equipment for 10min before bonding.
All bonding experiments were carried out in a vacuum hot press. The background vacuum before heating was 4×10-2Pa and the heating and cooling rates were 20℃/min. The bonding pressure was impacted on the surface of the specimens from the beginning of the experiment and kept constant. The bonding pressures of 4kPa were obtained by putting a dead weight on the top surface of the specimens, while 2MPa was impacted by a hydraulic system of the hot press.
Two kinds of stacking sequence of the master materials were involved. One was TiAl/Ti-Cu/Ti6242 and the other was TiAl/Cu-Ti/Ti6242 with reference to the difference of the stacking sequence of insert metals. The corresponding interfaces between TiAl and interlayer were denoted as TiAl/Ti-Cu and TiAl/Cu-Ti.
Interface microstructures were investigated by scanning electron microscopy(SEM). Composition analyses were carried out using energy dispersion X-ray spectrum(EDX).
3 RESULTS
Fig.1 shows only the morphologies of the interfaces of TiAl/Ti-Cu interface because no bonding defects are found at the interfaces between Ti6242 and interlayer. There are a few bonding defects(inclusions and voids) with relative large size at the interface, shown in Fig.1(a), while there are only several small defects at the interface, shown in Fig.1(b). The interface shown in Figs.1(c) and (d) has no defects. Based on the experimental results, conclusion can be drawn that the number of defects decreases with increasing bonding pressure, bonding temperature and holding time.

Fig.1 Interface morphologies of TiAl/Ti-Cu
EDX analysis reveals that the inclusions in Fig.1(a) contain high oxygen content (5%-24%, mass fraction). Moreover the inclusions are isolated in the interlayer, so conclusion can be deduced that the wettability between inclusions and liquid phase is poor.
4 DISCUSSION
4.1 Effect of TiAl surface
EDX analysis shows that there is an oxide layer with thickness of approximately 1μm on the surface of TiAl. As a result of the formation of the oxide layer, many vacancies will be left behind in TiAl beneath the surface. Certainly there are still a few discontinuous areas in the oxide layer. Fig.2(a) schematically shows the initial surface state of TiAl(the size of the void or vacancies have been enlarged). Such discontinuity will afford primary diffusion paths(shown in Fig.2(c) by dark arrows) for Cu atoms from Ti-Cu liquid phase into TiAl.
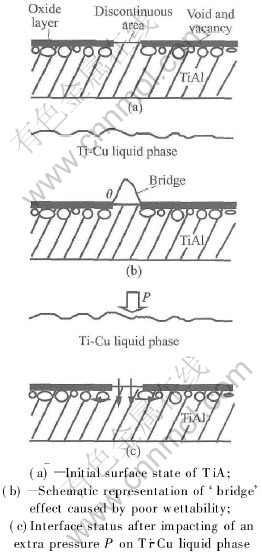
Fig.2 Effect of bonding pressure on ‘bridge’ behavior at discontinuous
Owing to the occurrence of oxide layer, the interface was changed from TiAl/Ti-Cu liquid phase to oxide layer/Ti-Cu liquid phase. As convinced by the experiments, the wettability between Ti-Cu liquid phase and the oxide layer is poor. So a so-called ‘bridge’ effect will appear evidently at the discontinuous area, which is shown schematically in Fig.2(b). Such a ‘bridge’ effect will retard the contact of the liquid phase to TiAl during bonding and form voids after cooling.
The primary surface status of TiAl has great effects on the diffusion process of Cu atom. There are two diffusion paths in TiAl for Cu atoms. The vacancies beneath the oxide layer will serve as quick diffusion paths for Cu atoms during bonding. So having diffused into TiAl at the discontinuous areas, Cu atoms will diffuse laterally with the aid of the vacancies. The other diffusion path for Cu atoms is to diffuse forward into the TiAl matrix. Meanwhile, some vacancies or voids will coalesce or aggregate to form visible voids. Such an interface structure is shown in Fig.1(a), in which the interlayer and TiAl are well bonded at the discontinuous areas where Cu atoms have longer diffusion distance than that at the other areas and spread laterally along the interface. Beneath the inclusions, some voids are visible.
4.2 Effect of experimental parameters
Bearing in mind that the ‘bridge’ effect and the diffusion behavior of Cu atoms have great effects on the interface evolution between Ti-Cu interlayer and TiAl. Any factor which abbreviates the ‘bridge’ effect or improves the diffusion process of Cu atoms will benefit the interface formation.
As mentioned above, the ‘bridge’ effect is mainly caused by the poor wettability between the oxide layer and Ti-Cu liquid phase. In order to improve the interface wettability, some measures should be taken. As a given temperature, the most practical and convenient approach is to apply a pressure on the liquid phase to force Ti-Cu liquid phase contact with TiAl in the discontinuous areas. The effect of bonding pressure on the interface status is shown in Fig.2(c). The ‘bridge’ effect can be abbreviated by impacting a pressure on the liquid phase. When the pressure is high enough, the ‘bridge’ effect will be vanished and good interface bonding can be obtained. Such an interface structure is shown in Fig.1(b).
Another measure method is to increase the bonding temperature. With increasing bonding temperature, the contact angle θ will decrease. Fig.3 schematically shows the effect of temperature on the ‘bridge’ behavior. If the interface is not wettable(see Fig.3(b)), the liquid phase will have a tendency to contract along the interface(shown by the arrows V in Fig.3) and strengthen the ‘bridge’ effect. If the interface is wettable(see Fig.3(c)), the liquid phase will obtain a tendency to spread on the surface, which helps make intimate contact between Ti-Cu liquid phase and TiAl at discontinuous areas and benefits to get sound bond between TiAl and interlayer. Such an interface structure is shown in Fig.1(c).
4.3 Formation of bonding defects
When Cu atoms diffuse laterally in TiAl with the aid of vacancies, the thermal expansion coefficient of the matrix will be changed, which leads to strong inner stress at the interface and makes the oxide layer detach from the matrix. The detached oxide layer will decompose during bonding and dissolve gradually into Ti-Cu liquid phase. So it becomes smaller and smaller in size with prolongation of holding time. When this process cannot fully complete, the oxide layer will be entrapped in the interlayer as inclusions which is shown typically in Figs.1(a) and (b). With the progress of diffusion process or the increase of the holding time, the voids will disappear and inclusions will dissolve into Ti-Cu liquid phase completely(see Fig.1(d)).
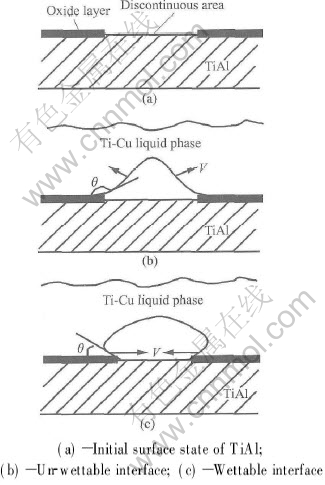
Fig.3 Effect of temperature on ‘bridge’ behavior in discontinuous areas
4.4 Effect of stacking sequence
When Cu foil is adjacent to TiAl, a liquid phase with high Cu-content will be formed at interface TiAl/interlayer before the whole foils turn into liquid state. This means that the inclusions at the interface have longer time to decompose or dissolve into Ti-Cu liquid phase in the joint of TiAl/Cu-Ti/Ti6242 than that in TiAl/Ti-Cu/Ti6242. Fig.4 shows the morphology of TiAl/Cu-Ti interface produced at the same experimental conditions as that of Fig.1(b). No inclusions and voids can be observed at this interface.

Fig.4 Morphology of TiAl/Cu-Ti interface
5 CONCLUSIONS
1) Defects locate at interface TiAl /interlayer when the bonding parameters are not optimized.
2) The surface status of TiAl has great effect on the interface evolution between TiAl and interlayer. A ‘bridge’ effect will be formed because of the oxide layer on the surface of TiAl. The diffusion behavior of Cu atoms in TiAl is strongly controlled by the vacancies beneath the surface of TiAl.
3) Bonding pressure is necessary because of the poor wettability between the oxide layer on TiAl and Ti-Cu liquid phase. Increasing bonding pressure or temperature or extending holding time helps get defect-free joint.
4) The interface structure is different if the joint is fabricated under the same conditions except for the stacking sequence of the insert metals.
REFERENCES
[1]Shirzadi A A, Wallach E R. Temperature gradient transient liquid phase diffusion bonding: a new method for joining advanced materials [J]. Science and Technology of Welding and Joining, 1997, 2(3): 89-94.
[2]Khan T I, Wallach E R. Transient liquid phase diffusion bonding and associated recrystallization phenomenon when joining ODS ferritic superalloys [J]. Journal of Materials Science, 1996, 31: 2937-2943.
[3]MacDonald W D, Eagar T W, Cieslak M J, et al. Transient liquid phase bonding processes [A]. The Metal Science of Joining, the Minearals [C]. TMS Warkendale Penn: Metals & Materials Society, 1992. 93-100.
[4]Kim J J, Park J W, Eagar T W. Interface microstructure of partial transient liquid phase bonded Si3N4-to inconel 718 joints [J]. Mater Sci Eng A, 2003, 344A: 240-244.
[5]Zhang J X, Chandel R S, Chen Y Z, et al, Effect of residual stress on the strength of an alumina-steel joint by partial transient liquid phase(PTLP) brazing [J]. Journal of Materials Processing Technology, 2002, 122: 220-225.
[6]Gale W F, Orel S V. A microstructural investigation of NiAl/Ni-Si-B/NiAl transient liquid phase bonds [J]. Journal of Materials Science, 1996, 31: 345-349.
[7]Gale W F, Guan Z. Transient liquid-phase bonding in the NiAl/Cu/Ni system—a microstructural investigation [J]. Metall Mater Trans A, 1996, 27A: 3621-3629.
[8]Moore T J, Kalinowski J M. Diffusion brazing NiAl with self-generated filler metal, high-temperature ordered intermetallic alloys V [J]. Materials Research Society Symposium Proceedings, 1993, 288: 1173-1178.
[9]Strum M J, Henshall G A, Flore N F, et al. Liquid-assisted diffusion bonding of NiAl [A]. Advanced Joining Technologies for New Materials II [C]. Miami, FL: AWS, 1994: 76-88.
[10]Butts D A, Gale W F, Zhou T. Transient liquid phase(TLP) bonding of TiAl-alloys: wettability, microstructure, mechanical properties and potential for automation [A]. The 6th International Trends in Welding Research Conference Proceedings [C]. Pine Mountain, GA: ASM International, 2003. 495-499.
[11]Fergus J W, Zhou T, Dang B, et al. Oxidation resistance of transient liquid phase(TLP)-bonded gamma titanium aluminide [A]. 6th International Trends in Welding Research Conference Proceedings [C]. Pine Mountain, GA: ASM International, 2003: 799-803.
[12]Gale W F, Orel S V. Microstructural development in NiAl/Ni-Si-B/Ni transient liquid phase bonds [J]. Metall Mater Trans A, 1996, 27A:-1925-1931.
[13]Gale W F, Wallach E R. Microstructural development in transient liquid-phase bonding [J]. Metall Trans A, 1991, 22A: 2451-2457.
[14]Gale W F, Guan Z. Transient liquid-phase bonding in the NiAl/Cu/Ni system—a microstructural investigation [J]. Metall Mater Trans A, 1996, 27A: 3621-3629.
[15]Gale W F, Abdo Z A M. Bulk-alloy microstructural analogues for transient liquid-phase bonds in the NiAl/Cu/Ni system [J]. Metall Mater Trans A, 1999, 30A: 3111-3124.
Received date: 2004-12-15; Accepted date: 2005-01-28
Correspondence: DUAN Hui-ping, Associate Professor, PhD; Tel: +86-10-82317064; E-mail: hpduan@buaa.edu.cn
(Edited by LI Xiang-qun)