Microscopic mechanism in solvent extraction of Au(CN)-2 by cationic surfactant
CHEN Jing(陈 景)1, HUANG Kun(黄 昆)1, YU Jian-min(余建民)1,WU Jin-guang(吴瑾光)2, SHI Nai(施 鼐)2, XU Guang-xian(徐光宪)2
(1. Institute of Precious Metals, Kunming 650221, China;
2. College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China)
Abstract: A new solvent extraction system of Au(Ⅰ) by surfactant CTMAB-TBP-inert diluent-Au(CN)-2-H2O was investigated and established. The infrared spectra and the conductivities of loaded organic phases containing Au(Ⅰ) were recorded stage by stage. It was reported that in the presence of salting-out agents the pencent extraction of Au(Ⅰ) decreased, and the conversion of emulsions from W/O to O/W happened in the extraction processed. The results demonstrate that the extraction process of Au(CN)-2 is carried out by two steps. First, an associated molecule between CTMA+ and TBP, as (C16H33)(CH3)3N+·H2O·O-P(OC4H9)3, is formed by dint of water molecule, then it will pull Au(CN)-2 into the organic phase based on the electro-neutrality.
Key words: surfactant; solvent extraction; Au(CN)-2 CLC number: TF804.2
Document code: A
1 INTRODUCTION
Since Mooiman, Miller et al[1, 2] reported their investigation in 1983, solvent extraction of Au(CN)-2 from alkaline cyanide solutions has been an attractive project. In the recent years, these studies have been more and more explored, and the representative papers are listed in Refs.[3-17].
The solvent extraction of Au(CN)-2 by surfactant CTMAB(cetyl trimithyl ammonium bromide) has been studied. It is different from the solvent extraction of Au(CN)-2 by quaternary ammonium salts that the first CTMAB is a sort of typical cationic surfactant with a longer carbon-hydrogen chain; the second, it was added into the extracted aqueous phase directly by the molar ratio with Au(CN)-2 and the third, the extraction organic phase consists of a sort of inert diluent (n-dodecane or sulphonated kerosene) and a sort of modifier with basic group (TBP or mixed-alcohol).
It is interesting, in this extraction system, Au(CN)-2 could be highly selectively and quantitatively extracted into the organic phases[18-21] . It is also important there are some new results, such as the formation of W/O or O/W microemulsions and their conversion, the disruption of emulsions, the variation of infrared spectra and conductivities of the organic phases containing gold and the influence of adding the salting out agents in aqueous phase.
In this paper we try to show the microscopic process in solvent extraction of Au(CN)-2 by cationic surfactant based on some new idea.
2 EXPERIMENTAL
2.1 Materials
Aurous solution was prepared by aurous potassium cyanide, KAu(CN)2, with AR grade, and the initial concentration was 5.077×10-3 mol/L containing Au 1.002g/L. Sulphonated kerosene was obtained by refining the commercial kerosene. Tri-butyl-phosphate(TBP), cetyl trimithyl ammonium bromide(CTMAB) and other chemicals used are of AR grade.
2.2 Determination of gold content
The gold contents in the raffinates and the organic phase were determined by Z-8000 Atomic Absorption Spectrograph or 722 Spectro-photometer.
2.3 Infrared spectroscopy
The infrared spectra of the organic phase in the ranges 670-3550cm-1 and 2850-8850cm-1 were obtained by using a SP100 Infrared Spectro-meter.
2.4 Conductance
The conductivities of the organic phase were determined by using a DDS-11A Conductometer at (25±0.5)℃.
2.5 Extraction systems
The six extraction systems in Fig.1 were investigated. The extraction system F was composed of five components, containing surfactant, modifier, inert diluent, Au(CN)-2 and water. It could be evolved either from the system A to B to D or A to C to E. The dosage and volume of components and the character of phenomena in the six systems are listed in Table 1.
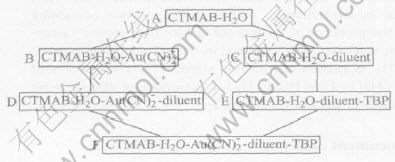
Fig.1 Evolvement of five element extraction system
3.1 System B: CTMAB-H2O-Au(CN)-2
Since the CTMAB aqueous solution was added into the NaAu(CN)2 solutions by mole ratio 1∶1 with Au, immediately , then it can be seen that the white exiguous precipitate was formed. This is due to the fact fact the body of Au(CN)-2 is bigger than that of Br-, while its surface charge density is much smaller, so the hydration of Au(CN)-2 is much weaker, and is easy to form the big neutral molecule with C16H33(CH3)3N+ according to Eqn.(1).
R(CH3)3N++Br-+Au(CN)-2 +xH2O= R(CH3)3N+·Au(CN)-2·xH2O↓+Br-(1)
where R represents the C16H33 group. In the aqueous solutions, the surfactant itself is easy to form the colloid owing to the twist of long chain alkyl, the precipitate formed in the reaction may be a sort of ion association among which the hydrophilic group is wrapped up, even some of water molecules carried in.
The white precipitate was altered out through the compact filter paper, and dried out by the vacuum drier, and then analyzed on the components of it. The results obtained show that the components of the precipitate are Au(CN)-2, CTMAB and H2O, and their mole ratio is 5.846∶7.309∶1, respectively. In the aqueous phase, as we have known, the molar of CTMAB was the same as the Au(CN)-2, so the gold contained in the precipitate is about 80% of the total gold content, and it indicates that Au(CN)-2 could only be precipitated by 80% in Eqn.(1).
3.2 System D: CTMAB-H2O-Au(CN)-2-inert diluent
The equal volume of inert diluent was added into the system C containing the white precipitate, then shaken, and laid aside for 24h, a coexistent three-phase system was obtained, as shown in Fig.2. The up-layer is the clear organic phase and the dow n-layer is clear aqueous phase, while the middle-layer is emulsion. The gold contents in the aqueous phase and organic phase were analyzed chemical method. The obtained data show that their gold contents are all quite low, which indicates that the Au(CN)-2 is mainly gathered in the white emulsion. This can be explained as that the precipitate in Eqn.(1), R(CH3)3N+·Au(CN)-2·xH2O, was unsoluble in the inert diluent. Even though some of its hydrophobic groups R— have been inserted in the organic phase, the hydrophilic end with positive charge and the counter ion Au(CN)-2 with negative charges as well as the water molecules enwrapped into the above two polar groups all yet cannot be extracted into the
Table 1 Dosage and volume of components and characters of these systems
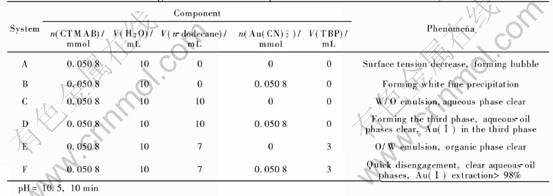
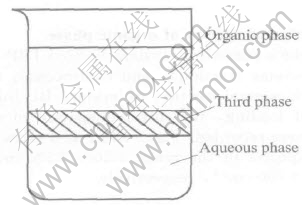
Fig.2 Formation of third phase of system D
organic phase, so the precipitate containing gold concentrated in the milky third phase of the middle-layer.
3.3 System F: CTMAB-H2O-Au(CN)-2-inert diluent-TBP
Since the equal volume of the organic phase of 50%TBP in n-dodecane was added into system B, shaken and then laid aside, the system quickly divided into two clear up and down layers. While if an amount of TBP was added into the system D directly, the milky third phase disappeared. The concentration of Au(Ⅰ) in the two aqueous phases are all dripped to 0.022g/L, and the one-run percentage extraction of gold are all about 98%. These can be explained that a sort of polar head-to-polar head associated molecule between the Lewis acid CTMA+ and the Lewis alkali TBP, or a sort of associated molecule by dint of the hydrogen bonding of water molecule was formed. The following facts will indicate that, the associated molecule formed mainly depends on the bridging of the hydrated water in R(CH3)3N+·OH2 or the hydrated water of the P-O bond in TBP.
The hydration energy released from the association will compensate the energy needed by stripping the weak hydrated layer of Au(CN)-2 , and the Au(CN)-2 will be extracted into the organic phase based on the electric neutrality principle.
Using 50%TBP in n-dodecane as the extraction organic phase, the extraction reaction can be expressed as
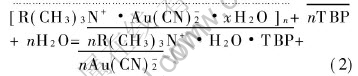
where the dotted line and the full line represent the aggregates in the third phase and the organic phase, respectively. Eqn.(2) shows that, TBP makes the associate between the surfactant and Au(CN)-2 decomposed. This is due to the fact that the hydrophilic group in the R(CH3)3N+·H2O·O-P(OC4H9)3 is enwrapped, and the unit positive charge is distributed over the several methylic hydrogens, easy to be solvated by water and TBP molecules, the surfactant loses the surface activity, unnecessary to array at the interface of the organic and aqueous phase, and also unnecessary to associate with the Au(CN)-2 in the organic phase. So the conductivity of the organic phase increases with the extraction stages.
3.4 System C: CTMAB-H2O-inert diluent
The equal volume of inert diluent, n-dodecane or sulfonated kerosene, was added into the CTMAB aqueous solution, and shaken in the separatory funnel, and then laid aside for the phase disengagement. We can see the down-layer aqueous phase is clear, but the up-layer organic phase is emulsive, that is, the W/O emulsion is formed. The W/O emulsion structure formed in the organic phase are shown in Fig.3.
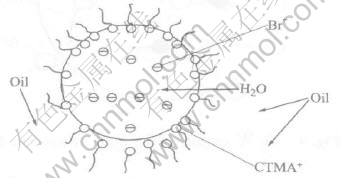
Fig.3 W/O emulsion structure formed in organic phase
It is thinkable for that, in fact, CTMAB is soluble in anhydrous inert diluent at some extent, and its solubility increases with the temperature; but if the organic phase is contacted with the CTMAB aqueous solution, the hydrophilic group of the surfactant will be inserted in the water as shown in Fig.3. On the other hand, the hydrophilicity of Br- is stronger and it has a strongly hydrated shell, so that it can not be extracted into the organic phase.
3.5 System E: CTMAB-H2O-inert diluent-TBP
A moderate amount of TBP was added into the system C, shaken, and then laid aside for a while, the up-layer of the system become clear, while the down-layer emulsive. Increasing the dosage of CTMAB to some extent, the interface of water-oil phases disappeared, the whole system is emulsive. This emulsion system is very stable, and not varied for one month.
As far as we know, this important result has not been reported by other authors elsewhere. We think the TBP in the extraction process does not serve the function of an assistant surfactant, but converts the W/O into the O/W emulsion.
Fig.4 shows the conversion process. Since
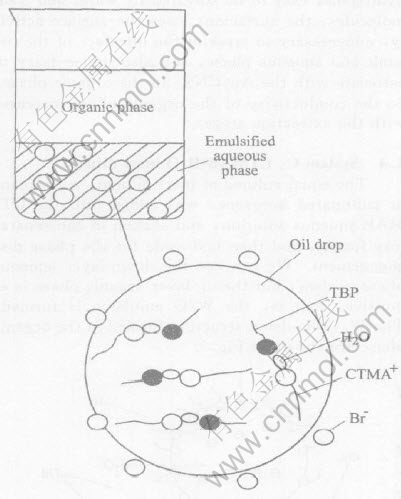
Fig.4 O/W emulsion formed in system E
TBP was added into system C, the hydrophilic end of the cation CTMA+ arrayed over the interface of oil-water phases will associate with the basic group P-O in TBP by virtue of the bridging of water molecules, and draw the ammonium group inserted in the water into organic phase. The positive charge in the organic phase will exclude with each other and make the organic phase to divide into many of small oil drops with positive charge. The Br- was absorbed around the surface of the small oil drops, and so the stable O/W emulsion was formed. It was observed under the microscope with the dyeing method. We could see that the colorful water is a continuous phase.
Since the aurous solutions containing Au(CN)-2 was added into the system E, for the lower face charge density of Au(CN)-2, the Au(CN)-2 would be pulled into the organic phase prioritically and the organic phase became electroneutrality. The driving force of Au(CN)-2 into the oil drops is similar to the electric-osmotic force. The electroneutral oil drops quickly, combines with each other, grows up, and becomes system F that is clear and diaphanous water-oil phases.
The O/W emulsive phase of system E can also be disrupted by adding NaClO4 or NaSCN solution. This is due to the lower face charge density of ClO-4 and SCN- than Br-, easy to be pulled into the tiny oil-drops to neutralize the positive charge in them.
3.6 Infrared spectra of organic phase
Take a portion of system F(50%TBP-n-dodicane, volume fraction), and continuously contacted with several portion of system B, for every stage of loading, the infrared spectra of organic phase were recorded. Fig.5 and Fig.6 show the infrared spectra in the range 1200-2200cm-1 and 3100-3700cm-1, respectively.
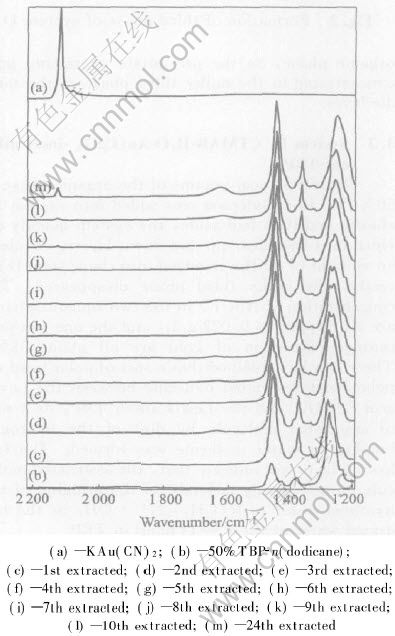
Fig.5 Infrared spectrum of stage-by-stage loaded organic phase in range of 1400-2200cm-1
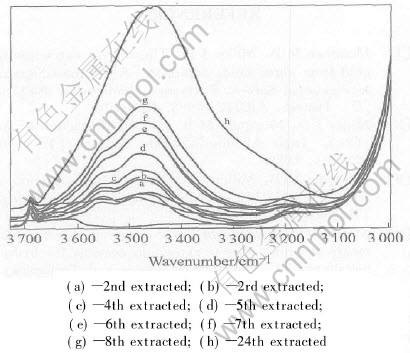
Fig.6 Infrared spectrum of stage-by-stage loaded organic phase in range of 3100-3700cm-1
In Fig.5, it can be seen that, the intensity of [JG(]C[ZJSX,Y]N[JG)] stretching band at 2141cm-1 increases with the extraction steps, this is due to the fact that the loaded Au(CN)-2 content in the organic phases increases with the extraction steps. And the 1284cm-1 band of P-O stretching mode moves to lower frequence, 1264cm-1, but the band area does not vary obviously. This may be due to the extractive cation among TBP, CTMA+ and H2O, named as R(CH3)3N+·OH2·O-P(OC4H9)3, is formed in the system. We know that the O-P group in TBP is H-bonding associated with H2O in the system, while the positive charges in quaternary ammonium cation may be distributed over the methylic hydrogens by the induction effect, so that the methylic hydrogen with δ+ charge will associate with the oxygen in the water.
With the increase of the extraction stages, the concentration of the associated cation, [R—(CH3)3N+·OH2· TBP], in the organic phase stepwise increases, and the frequence shift of P-O group stretching mode became more and more obvious.
For the question how many H2O and TBP molecules were associated with a quaternary ammonium cation, it is necessary to study further. Here we postulate all as one for H2O and TBP molecule.
As shown in Fig.6, with the increase in the extraction stages, the intensities and the area of —OH stretching band at 3462cm-1 became more and more large due to the increase of the H2O content in the organic phases, while it has been thought the coming from the increase of the surfactant molecules extracted into the organic phases. This fact supports the precious discussion on the extraction mechanism. We have more detailed explanation about the infrared spectra of the organic phase[22, 23].
3.7 Conductivities of organic phase
Fig.7 shows the conductivities of the loaded organic phase in different extraction steps. It can be seen that the conductivies increase with the gold concentration in the organic phases and reach a maximum value at 11.24g/L gold concentration, and then decrease. It may be due to the fact that, at beginning, the Au(CN)-2 extracted in the organic phases existed in free state, as just shown in Eqn.(2), the lower gold concentration in the organic phase made the lower conductivity, and increased with the gold concentration in the organic phase until the occurrence of the associated cation, [R(CH3)3N+·OH2·O-P(OC4H9)3], in the organic phases or the formation of W/O microemulsion packed Au(CN)-2, then immediately decreased , and on this, we can see the organic phase separated into two layers, a hight layer and heavy layer.
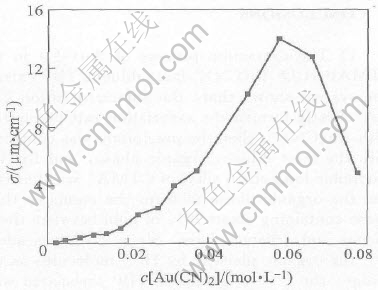
Fig.7 Conductivities of stage-by-stage loaded organic phase
3.8 Salting out effects in aqueous phase
Adding Li, Na, K, Ca and Mg chloride into system A , their effect on the gold extraction is shown in Fig.8.
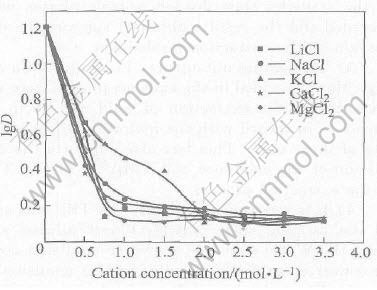
Fig.8 Influence of salting out agents on distribution ratio of gold
In Fig.8 we observe the addition of the these salting out agents is unfavorable to the gold extraction. When the concentration of these chlorides in the aqueous phases is higher than 0.75mol/L, the percent gold extraction is decreased sharply. Their influence sequence for the percent gold extraction is Li+>Na+>K, and Mg2+>Ca2+. That is just the sequence of decreasing hydration of these ions due to the increase of their ion radii. This means the addition of the salting out agents decreases the water activity and is unfavorable to the formation of the associated cation, CTMA+·H2O·TBP, which is bridged by H2O molecule, so the percent gold extraction is decreased, which is the same as that reported on solvent extraction of Zr(Ⅳ) in hydrochloride acid system[24].
4 CONCLUSIONS
1) The extraction process of Au(Ⅰ) in the CTMAB-H2O-Au(CN)-2-inert diluent-TBP extraction system shows that, the sufactant cation CTMA+ firstly formed the association salt precipitate with Au(CN)-2; then the precipitate was contacted with the inert diluent organic phase, and the hydrophobic long-chain alkyl of CTMA+ was inserted into the organic phase to form the emulsive third phase containing about 98% of gold between the aqueous and organic phase. Since TBP was added into the organic phases, by H2O molecules as the bridge, the P-O group of TBP associated with the hydrophilic end of CTMA+ to form the large cations. Perhaps the hydrophilic part located in the middle of the large cation lost the surface activity, so that the large ion were easy to be dissolved into the inert diluent; and then Au(CN)-2 with lower face charge density will be drawn in the organic phase based on the electroneutrality.
2) The infrared spectra and the conductivities of the stage-by stage loaded organic phases were recorded and the results obtained support the discussion on the extraction mechanism.
3) The salting out agents, Li, Na, K, Ca and Mg chloride, added in the aqueous phases were unfavorable to the extraction of gold and their sequence is in accord with the hydration effect intensity of those ions. This fact also supports the discussion of the existence of CTMA+·H2O·TBP in the extraction process.
4) It is reported that, since the TBP was added the cationic surfactant-H2O-inert diluent system, the W/O(water/oil) reverse emulsion could be converted to the O/W(oil / water) emulsion.
5) The main driving force in the extraction of Au(CN)-2 by cationic surfactant CTMAB(or other quaternary ammonium salts ) is the association among CTMA+, H2O and TBP in the inert diluent, which lowers the energy of the system due to the evolution of the hydration energy, and the large cation CTMA+·H2O·TBP will pull Au(CN)-2 with lower hydration energy into the organic phase by the coulombic force.
REFERENCES
[1]Mooiman M B, Miller J D. The solvent extraction of gold from aurocyanide solutions [A]. Proceedings of International Solvent Extraction Conference, ISEC83 [C]. Denver: AICHE, 1983. 530-531.
[2]Miller J D, Mooiman M B. Solvent Extraction of Au(CN)-2 from Alkaline Cyanide Solution [P]. US 4774003, 1988.
[3]Mooiman M B, Miller J D. The solvent extraction of Au(CN)-2 [J]. Mineral and Metallurgical Processing, 1984, 53: 157-162.
[4]Miller J D, Mooiman M B. A review of new developments in amine solvent extraction systems for hydrometallurgy [J]. Separation Science and Technology, 1984-85, 19(11-12): 895-909.
[5]Mooiman M B, Miller J D. The chemistry of gold solvent extraction from cyanide solution using modified amines [J]. Hydrometallurgy, 1986, 16: 245-251.
[6]Riveros P A. Studies on the solvent extraction of gold from cyanide media [J]. Hydrometallurgy, 1990, 24: 135-156.
[7]Alguacil F J, Caravaca C, Martinez S. Extraction of gold from cyanide or chloride media by cyanex 923 [J]. Journal of Chem Technol Biotechnol, 1998, 72: 339-346.
[8]Alguacil F J, Hernandez A, Luis A. Study of the KAu(CN)2 -amine amberlite LA-2 extraction equilibrium system [J]. Hydrometallurgy, 1990, 24: 157- 168.
[9]Caravaca C, Alguacil F J, Sastre A, et al. Extraction of gold(I) cyanide by the primary amine tridecylamine [J]. Hydrometallurgy, 1996, 40: 89-97.
[10]Caravaca C, Alguacil F J, Sastre A. The use of primary amines in Gold(I) extraction from cyanide solutions [J]. Hydrometallurgy, 1996, 40: 263-275.
[11]Caravaca C, Alguacil F J. Gold(I) extraction equilibrium in the system KAu(CN)2 primene JMT sulphate-xylene [J]. Hydrometallurgy, 1992, 31:257-264.
[12]Caravaca C, Alguacil F J. Extraction of gold from cyanide aqueous-media by primene-JMT [J]. Hydrometallurgy, 1994, 35: 67-78.
[13]Mi1ler J D, Wan R Y, Mooiman M B, et al. Selective solvation extraction of gold from alkaline cyanide solution by aykyl phosphorous esters [J]. Separation Science and Technology, 1987, 22(2/3): 487-502.
[14]Alguacil F J, Caravaca C, Coba A, et al. The extraction of gold(I) from cyanide solutions by the phosphine oxide cyanex 921 [J]. Hydrometallurgy, 1994, 35: 41-52.
[15]Alguacil F J, Caravaca C, Martinex S, et al. The phosphine oxides cyanex 923 and cyanex 925 as extractants for gold(I) cyanide aqueous solutions [J]. Hydrometallurgy, 1994, 36: 369-384.
[16]Kordosky G A, Sierakoski J M, Virnig M J, et al. Gold solvent extraction from typical cyanide leach solutions [J]. Hydrometallurgy, 1992, 30: 291 -305.
[17]Mooiman M B, Miller J D. The chemistry of gold solvent extraction from alkaline cyanide solution by solvating extractants [J]. Hydrometallurgy, 1991, 27: 29-46.
[18]Zhu L Y, Chen J, Jing Y Q, et al. Solvent extraction of Au(CN)-2 when additive added into aqueous phase [J]. The Chinese Journal of Nonferrous Metals, 1996, 6(2): 229-232.(in Chinese)
[19]Pan X J, Chen J. Solvent extraction of gold(I) when additive added into aqueous phase [A]. The National Metallurgical Physical Chemistry Society. Proceedings of 1996 the National Symposium on Metallurgical Physic-chemistry [C]. Kunming: The National Metallurgical Physical Chemistry, 1996. 206-213.(in Chinese)
[20]Chen J, Zhu L Y, Jing Y Q, et al. Solvent extraction of gold cyanide with tri-butyl-phosphate and additive added into aqueous phase [A]. Manziek Rohm & Haas Company. Precious Metals 1998 [C]. Toronto, Ontario, Canada: International Precious Metals Institute, 1998. 65-74.
[21]Huang K, Chen J. Solvent extraction of gold(I) in mixed alcohol-diluent-surfactant-Au(CN)-2 system [A]. Deng D G. Proceedings of the International Symposium of Precious Metals, ISPM99 [C]. Kunming: Yunnan Science and Technology Press, 1999. 283-288.(in Chinese)
[22]Yan W F, Ma G, Weng S P, et al. Solvent extraction mechanism of Au(CN)-2 by surfactant from the alkali cyanide solution [J]. Acta Scientiarum Naturalium Universitatis Pekinensis, 2000, 36(2): 461-466.
[23]Yan W F, Ma G, Chen J, et al. Mechanism of Au(CN)-2 solvent extraction by surfactant [J]. Spectroscopy and Spectral Analysis, 1999, 19(6): 806-810.
[24]Yu S Q, Chen J Y. Discussion on salting-out effect for two different solvent extraction mechanism [J]. Acta Metallurgical Sinica, 1984, 20(6): B342-351.
(Edited by LONG Huai-zhong)
Foundation item: Project(29876016) supported by the National Natural Science Foundation of China; Project(9808K) supported by the Yunnan-Peking University Research and Development Cooperation Foundation
Received date: 2004-05-08; Accepted date: 2004-10-01
Correspondence: CHEN Jing, Professor, Academician of CAE; Tel: +86-871-5133963; E-mail: Jingchenipm@hotmail.com