Article ID: 1003-6326(2005)03-0504-06
Formation mechanism of amorphous
Ni-Fe-P alloys by electrodeposition
Received date: 2004-08-31; Accepted date: 2004-12-20
GAO Cheng-hui(高诚辉)
(College of Mechanical Engineering, Fuzhou University, Fuzhou 350002, China)
Abstract: The formation mechanism of the amorphous Ni-Fe-P coating was studied by analysis of the forming thermodynamics, dynamics, and crystallography of the amorphous alloy. The results show that, in the initial stage of deposition a thin “crystal epitaxial growth” layer first forms, and then transforms to amorphous gradually. The cross section in Ni-Fe-P coatings by electrolytic etching exhibits a banded structure of alternate dark and light bands. It is proposed that the banded structure is caused by a change in the P content with thickness, which is due to alternated depletion and enrichment of [OH-] in the diffusion layer resulting from the generation and evolution of hydrogen gas. The amorphous Ni-Fe-P coating will be formed in proper composition, high nucleation rate and strongly hindered growth of the crystal nucleus. Amorphous Ni-Fe-P alloys form as islands, and grow up by layer.
Key words: Ni-Fe-P Coating; amorphous alloy; formation mechanism; electrodeposition CLC
number: TG139.8 Document code: A
1 INTRODUCTION
The amorphous alloys have been developed fast and used extensively in recent 20 years[1]. They possess many specific properties[2-4] and are often used as functional materials, for example, the amorphous Ni-P alloy has been applied to automobile[5], aircraft, computer, electronics, engineering, food processing, petroleum[6]. Among those the most attractive are the amorphous alloys of Fe family-semimetal system. The Ni-Fe-P is one of them. It has soft magnetic properties[7] and high wear resistance[8]. It can be used as magnetic recording materials[9].
Due to the lack of the knowledge of the amorphous structure, the formation mechanism of amorphous alloys during electrodepositing is not very clear. In the electrodeposition of Ni-P coating it is only concerned with the reduction precipitation of Ni, P, and H[10]. Aoki et al[11] dealt with electroless Ni-P films in the initial stage of deposition by TEM. Nee and Weil[12] studied the banded structure of Ni-P electrodeposits. ZHANG et al[13] studied the electrochemical behavior of Ni-P alloy deposition. RAO et al[14] discussed the formation mechanism of electroless Ni-B alloy coating. SUN et al[15] studied the behaviors of the initial depositing stage of electroless Ni-Co-P plating. YU et al[16] discussed the deposition mechanism of electroless amorphous Ni-W-P alloy. Watanabe[17] discussed the formation mechanism of amorphous alloys. The practical value of the amorphous coating lies in its unique properties that depend on the growing process of the coating in a great degree. The study on the formation mechanism of amorphous coating not only enriches the theory of amorphous alloy, but also has an important practical significance. In this paper the formation mechanism of the amorphous Ni-Fe-P coating is investigated.
2 EXPERIMENTSAL
The components of the bath and the technological parameters were the same as those in Ref.[7]. The electron spectrum for chemical analysis (ESCA) was tested by PHI550ESCA/SAM multifunction electronic spectrometer. After the ESCA full spectrum detection and being cleaned by Ar+, the multiple analyses about the characteristic energy of Ni, Fe, P was made by a computer, and then the detailed spectrum of each element can be gained. The samples for transmission electron microscopy were prepared by electroplating Ni-Fe-P amorphous coating on the polished thin copper sheet, then dissolving the copper sheet with chromic acid and cleaning it by distilled water. Observation and electro diffraction were performed by the JEM-1200EX/S transmission electron microscope. The surface profile and the cross section structure of amorphous Ni-Fe-P coating were observed by the HITACHI X-650 scanning electron microscope. Characteristic X-ray diffraction was used to determine the variation of phosphorus contents in the direction of thickness.
3 RESULTS
3.1 ESCA analysis
The ESCA detailed spectra of amorphous Ni57-Fe25P18 alloy coating are shown in Fig.1. The corresponding binding energies of two peaks, the Ni2p1/2 and Ni2p3/2, are 870.0eV and 852.7eV respectively, and Fe2p1/2, Fe2p3/2, are 723.2eV and 710.4eV respectively, while that of P2p is 129.6eV. It is shown that the elements are in nullvalent state comparing these binding energies with those of the ESCA standard pattern.
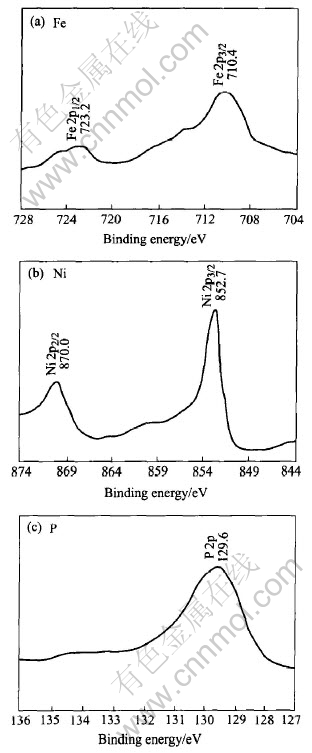
Fig.1 ESCA detailed spectra of elements in amorphous Ni-Fe-P coating
3.2 TEM observation and electron diffraction analysis
Fig.2 shows the TEM micrographs and diffraction patterns of Ni-Fe-P films electrodeposited at different time. The image at 10s shows light graininess, whereas that diffraction pattern shows spottiness. This indicates that in the initial stage of deposition, the crystal is formed at first. In the image at 20s there are a lot of light circles with different sizes. The diameter of the small one is only several nanometers, while that of the large one is about 20μm. The pattern displays diffraction rings. The light point might be some micro-crystals. The coating consists of micro-crystal and amorphous. With the increasing of the electroplating time the image at 120s shows many black crumbs or circles. Their diameter is about several nanometers. The diffraction pattern displays two scrappy halos. The second one is very vague. The

Fig.2 TEM micrographs and diffraction patterns of Ni-Fe-P films in initial stage of deposition
diameters of the two halos are measured to be 25.0mm and 43.0mm respectively. The diffraction pattern is the same as that of the typical amorphous alloy. From above analysis, we consider that the coating is electrodeposited from crystal, microcrystal and amorphous, and to amorphous gradually.
3.3 Structure and surface topography analysis of coatings
Fig.3(a) shows the microstructure of the cross section (normal to coating surface) of the Ni-Fe-P coatings. It exhibits a structure of alternate dark and light bands parallel to the surface. The widths of the bands are different. Some dark bands are very pronounced while others are fainter. The light bands are generally wider than the dark ones. The structure is laminated in three-dimensional space. There is a thin transitional layer at the interface of the coating and substrate, which is seen as columnar crystals in SEM micrograph, as shown in Fig.3(b).
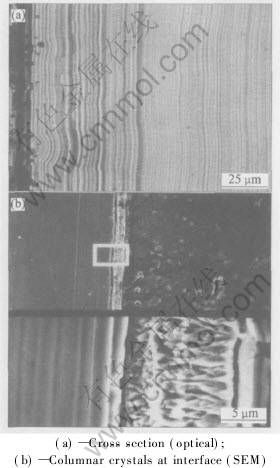
Fig.3 Microstructures of coating layers
(galvanic attack)
Fig.4 shows the characteristic X-ray profile of the phosphorus element on the cross section of the coatings. The P content fluctuated along the cross section. This irregularly periodic variation corresponds relatively well to the band structure. It is seen that the larger peaks in the phosphorus content generally correspond to light bands. It is the variation of P content that causes selective corrosion during electrolytic etching. This indicates that the co-deposition of phosphorus exhibits irregularly periodic variation during electroplating.

Fig.4 Characteristic X-ray radiation profile of phosphorus content
Fig.5 shows the surface micrographs of Ni-Fe-P coatings deposited at different contents of NaH2PO2·H2O. A lot of granular convexities can be observed on the surface clearly. Their grain sizes reduce with increasing phosphorus content. Therefore the brightness increases macroscopically[4].

Fig.5 Surface micrographs of amorphous Ni-Fe-P coatings with different P contents(SEM)
4 ANALYSES AND DISCUSSION
4.1 Crystal epitaxial growth effects
The results above show that in the initial stage the amorphous does not deposit on the substrate directly, but a thin transitional crystal layer is formed at the interface of the coating and substrate. It is then deposited from crystal→microcrystal and amorphous→amorphous gradually. The transitional layer is called “Crystal epitaxial growth” layer.
Since there exist many defects such as dislocation on substrate surface, Ni and Fe atoms easy to be reduced are affected by force field of substrate surface, and tend to be combined into existing lattice position on the surface as an electro-crystallization mechanism. The crystal grains of new deposit become small gradually. With the appearance of P co-deposition and increase of P content, amorphous appears in the coating, and the structure changes from a mixture of crystal and amorphous to amorphous gradually. Meanwhile there are no crystal defects such as dislocation in amorphous alloys. The deposition can not grow by the dislocation growing mechanism. According to the structure character of the amorphous alloys composed of short-range order colonies, it is considered that the absorbed atoms gather to form short-range order colonies similar to crystal nucleus during electrodeposition, but the growth of them is confined. The coating growth only depends on forming new short-range order colonies continuously. Thus coating is piled up totally by short-order colonies.
4.2 Formation of amorphous coating
The forming conditions of amorphous coating are reviewed in Ref.[17]. According to the forming energy theory of amorphous alloys of the Fe group metal-semimetal system, the more approaching to the eutectic composition, the smaller the forming energy is, the easier an amorphous structure forms. It is shown that the P element does not exist in the coating as a compound by ESCA analysis. Sadoc et al[18] consider that 2P atoms are never first neighbours according to the results of partial interference and radial distribution function, that is, there are no adjacent double P-P atoms in the amorphous Ni-P type alloy. It has a tetrahedral close packing(TCP) structure with P atoms spreading out in this matrix without long-range order and surrounded by 9 metallic atoms. Ni-Fe-P amorphous alloy is similar to Ni-P amorphous alloy. During the deposition of amorphous Ni-Fe-P alloy, P atoms have stronger chemical affinity with Ni and Fe atoms and produce chemical action with them, so atom colonies are formed with centering P atoms enveloped in Ni and Fe atoms. These atom colonies destroy the long-range order degree in the electrodeposited structure of Ni and Fe. The higher the P content is, the greater the disorder degree of the coating and the more percentage of amorous are. So, if the concentration of NaH2PO2 in the bath is low, it only can get microcrystalline layer, while amorphous structure appears when the P contents are beyond a certain value, meanwhile the coating presents a mixed state of crystalline and amorphous. However, when the co-deposited P content approaches to the eutectic composition, the coating presents as all amorphous state.
On the other side, P atoms have stronger chemical affinity with Ni and Fe atoms and produce chemical action with them. It seems to mean that it is easy to form the metallic compound. However even if the coating has the same composition as metallic compound, there are no metallic compounds discovered in the electrodeposited Ni-Fe-P alloy. Generally, the grain size of metallic compound is larger, and the structure is more complex. The metallic compound composed of P, Ni and Fe has a complex structure. One of its crystal cell parameters is approaching to or over 1 nm. The different type atoms must be arranged to form a complex lattice strictly, and the crystal formed with long-range order must be composed of scores crystal cells at least. The reduced atoms during electroplating want to enter their positions in this complex lattice, which depend on the diffusion of atoms. It needs great activation energies to finish the diffusion. However, the electroplating temperature is low, at which it is impossible to require the atoms to diffuse as the complex lattice of metallic compound. As they cannot span over the barrier of activation energy needed by crystallization, they can only keep the amorphous state in the short-range order. Here, diffusion process is regarded to be the factor impeding the ordered atom colonies to grow.
During electroplating, the metals must be reduced and precipitated under certain overpotential in order to overcome the energy barrier for deposition. According to the precipitating theory of the electrocrystallization[19], the accumulation of absorbed atoms becomes crystal nucleus or redissolves in solution below the critical size, which depends on the oversaturation of absorbed atoms on the growing surface, or the overpotential of electrocrystallization. The larger the overpotential, the smaller the critical size of crystal nucleus and the nucleating power, and the greater the nucleating rate. If we regard the amorphous as the limit of ultramicrocrystal, in order to precipitate amorphous, the overpotential of crystallization must be great enough to let the critical size lie in the shot-range order scope of amorphous alloy, and the growth of these shot-range order colonies is inhibited to a great extent. The order colonies have not enough time to grow, or the growing rate is far smaller than the nucleating rate, so the coating growth only depends on forming many new short-range order colonies continuously and rapidly[10].
As for electrodepositing the Ni-Fe-P alloy, when the contents of Ni, Fe and P elements approach to the eutectic composition, the crystallization as a metal or an exchanged solid solution is destroyed for the stronger chemical affinity between element P and Ni, Fe. At the lower bath temperature, however, the energy is not great enough to span the activation energy barrier for diffusion of atoms as the structure of metallic compound. Consequently the metastable amorphous structure is formed and maintained with centering P atoms enveloped in Ni and Fe atoms.
4.3 Formation of laminated structure
The surface profile of Ni-Fe-P coatings displays many granular convexities, the micrograph of cross section exhibits a banded structure that is laminated in three-dimensional space. So, it can be considered that the amorphous Ni-Fe-P coating grows up by layer after forming many islands. The analysis result of P distribution shows that the banded structure in Ni-Fe-P coating is caused by the changes in the P content with deposit thickness. The etchant that reveals the banded structure results in galvanic attack where regions of different phosphorus content are adjacent. According to Ref.[14] the formation of banded structure in Ni-P coating is related to alternate metal ion depletion and enrichment in the diffusion layer resulting from hydrogen evolution, that is, the cyclical change in P content is caused by the cyclical change in Ni content during deposition.
We consider that cyclical change of P content is related to the codeposition of element P in cathode surface. During electrodepositing Ni-Fe-P alloy, the following reducing reactions in cathode may occur:
Ni2++2e=Ni(1)
Fe2++2e=Fe(2)
2H++2e=2H=H2(3)
H2PO-2+e=2OH-+P(4)
In addition, the reducing reaction about P on cathode surface can also proceed by the means of non-electrochemistry, which is put forward in electroless plating theory[12]:
H2PO-2+H=H2O+OH-+ P(5)
or
H2PO-2+Ni=NiOH+OH-+ P(6)
From Eqns.(4), (5) and (6) we can discover that OH- ions appear all with reduction of phosphorus, no matter it is reduced by the means of electrochemistry or non-electrochemistry. As the concentration product of [OH-] and [H+] is a constant in aqueous solution, the cathode reduction of P is obviously influenced by [OH-] or pH value of bath. When pH value is low, the concentration of [H+] is high and the concentration of [OH-] is low, which benefits the reducing deposition of element P. The change of [OH-] or [H+] will affect P co-deposition.
During electrodepositing the Ni-Fe-P alloy, there appear reduction and evolution of hydrogen, which leads to the decrease of [H+] and the increase of [OH-] near the cathode. It will be unfavorable to reduction and deposition of phosphorus. However [H+] in the whole solution is higher than that nearby the surface of cathode, so the hydrogen evolution causes the ion in the solution to diffuse and convect, in order to supply the consumption of H+ nearby the cathode. It makes [H+] nearby the cathode be supplied and approach to [H+] in the whole. So the co-deposition of P increases again.
From above analysis, we can deduce that from the generation or the formation to the evolution of hydrogen gas out of solution, the [H+] in the diffusion layer will change periodically, which causes [OH-] in the diffusion layer to produce the alternated depletion and enrichment, hence leads to the cyclical change in co-deposition of phosphorus, that is, the cyclical change in the phosphorus content. As a result, the cross section etched by galvanic attack exhibits a banded structure of alternate dark and light bands.
5 CONCLUSIONS
1) During electrodepositing the amorphous Ni-Fe-P alloy, a thin transitional crystal layer is formed at the interface of the coating and substrate. It is deposited from crystal, microcrystal and amorphous, and to amorphous gradually. The production of the “crystal epitaxial growth” layer is related to the influence of substrate material in the initial stage of deposition and the reduction and deposition of Ni and Fe easily.
2) The larger chemical affinity of P atoms with Ni and Fe atoms destroys the normal crystallization of Ni and Fe and the energy is not great enough to span the activation energy barrier for diffusion of atoms as the structure of metallic compound at lower bath temperature. Consequently the metastable amorphous structure is formed with centering P atoms enveloped in Ni and Fe atoms.
3) Amorphous Ni-Fe-P alloys form as islands, and then grow up by layer. This laminated structure is related to the irregular cyclic variation of P content with deposit thickness.
4) The cross section in Ni-Fe-P coatings etched by galvanic attack exhibits a banded structure of alternate dark and light bands. It is proposed that the banded structure is caused by a change in the P content with thickness, which is caused by alternated [OH-] in the diffusion layer depletion and enrichment resulting from the generation and evolution of hydrogen gas.
REFERENCES
[1]GAO Cheng-hui. Plating of Amorphous Alloys and Their Properties[M]. Beijing: Science Press, 2004. 453. (in Chinese)
[2]Watanabe T. Amorphous plating[J]. Kinzoku Hyomen Gijutsu, 1987, 38(6): 210-218. (in Japanese)
[3]Nanayan R, Mungole M N. Hardness control in electrodeposited nickel-phosphorus coating[J]. Metal Finishing, 1985, 83(1): 55-57.
[4]GAO Cheng-hui, ZHOU Bai-yang. Structure and properties of electrodeposited Ni-Fe-P alloys with various compositions[J]. The Chinese Journal of Nonferrous Metals, 1996, 6(3): 81-85. (in Chinese)
[5]DiBari G A. Some automotive applications of electroless nickel coatings[J]. Metal Finishing, 1983, 81(12): 31-32.
[6]Baudrand D W. Trends in electroless nickel plating and look at the future[J]. Plating and Surface Finishing, 1983, 70(12): 24-26.
[7]GAO Cheng-hui, ZHOU Bai-yang. Effects of the composition of electrodeposited Fe-Ni-P alloy on the thermostability and magnetic properties[J]. J Mater Sci Technol, 1997, 13(2): 137-140.
[8]GAO Cheng-hui. Effects of electroplating specifications on the hardness and wear resistance of Fe-Ni-P coatings[J]. Materials Protection, 1996, 29(9): 4-6. (in Chinese)
[9]Osaka T, Homma T, Saito K, et al. Co-based soft magnetic films produced by electroless deposition[J]. J Electrochem Soc, 1992, 139(5): 1311-1314.
[10]GAO Cheng-hui. Fe group-metalloid amorphous alloy plating[J]. Materials Protection, 1991, 24(1): 7-11. (in Chinese)
[11]Aoki K, Takano O, Ishibashi S, et al. An electron microscopic study of electroless Ni-P films in the initial stage of deposition[J]. Kinzoku Hyomen Gijutsu, 1977, 28(1): 12-17. (in Japanese)
[12]Nee C C, Weil R. The banded structure of Ni-P eletrodeposits[J]. Surface Technology, 1985, 25(1): 7-15.
[13]ZHANG Qi-fu, TU Fu-zhou. Studies on the mechanism of amorphous nickel-phosphorus alloy electrodeposition[J]. Electroplating & Finishing, 2001, 20(1): 1-6. (in Chinese)
[14]RAO Qun-li, WANG Hao-wei, FAN Xiao-lan. Morphology and formation mechanism of electroless Ni-B alloy coating[J]. Journal of Shanghai Jiaotong University, 2003, 37(12): 1965-1968. (in Chinese)
[15]SUN Hong-fei, WU Yi-yong, SHAO Qian, et al. Behaviours of initial depositing stage of electroless Ni-Co-P plating[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(1): 81-84. (in Chinese)
[16]YU Su-fen, LEI Jun, FAN Wei-guang, et al. Preliminary study on the deposition mechanism of electroless Ni-W-P alloy[J]. Materials Protection, 2001, 34(12): 18-19. (in Chinese)
[17]Watanabe T. The formation mechanism of amorphous alloys by plating[J]. Hyomen Gijutsu, 1989, 40(3): 375-380. (in Japanese)
[18]Sadoc J F, Dixmier J. Structural investigation of amorphous CoP and NiP alloys by combined X-ray and neutron scattering[J]. Mat Sci Eng, 1976, 23: 187-192.
[19]CHA Quan-xing, et al. Introduction to the Dynamics in Electrode Process (3rd ed)[M]. Beijing: Science Press, 2002. 6. (in Chinese)
(Edited by YUAN Sai-qian)
Correspondence: GAO Cheng-hui, Professor, PhD; Tel: +86-591-87893270; Fax: +86-591-87892531; E-mail: gch@fzu.edu.cn