J. Cent. South Univ. Technol. (2010) 17: 45-49
DOI: 10.1007/s11771-010-0009-3 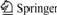
Preparation of lactic acid from glucose in ionic liquid solvent system
HUANG Jia-ruo(黄嘉若), LI Wen-sheng(李文生), ZHOU Xiao-ping(周小平)
College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: A new reaction system was designed to economically convert glucose to lactic acid environment-friendly. Hydrophobic ionic liquids were chosen as solvent that can promote the decomposition reaction of glucose, and the catalytic performance of the solid bases was evaluated. Both the reaction temperature and time can affect the yield of lactic acid. A high yield (97%) of lactic acid was achieved under the optimal reaction condition. The 1H NMR spectra and HPLC-MS were used to identify the formation of the lactic acid and variations of ionic liquid. It is found that ionic-liquids have a unique solvent effect for glucose and bases. Water can be used as solvent to extract calcium lactate. This shows a great potential of hydrophobic ionic liquids in the solid bases catalyzed reaction that is limited by the weak solubility of solid bases in organic and water solution.
Key words: glucose; lactic acid; ionic liquid; solid base; catalysis
1 Introduction
A sustainable future for the chemical industry requires feedbacks based on the renewable rather than steadily depleting resources. Inability to effectively transform five or six-carbon carbohydrates into building blocks derived from the nature is the major barrier towards this challenging goal. Both fructose and glucose, containing six carbon atoms, are potential feedbacks for this purpose, and recently efforts have focused on converting them to lactic acid, a chemical commodity. Some researchers reported similar processes such as converting glucose into 5-hydroxymethylfurfural (HMF) in a two-phase reaction system [1]. It is well known in sugar chemistry that the lactic acid is a typical base catalyzed product from carbohydrates [2-7]. Lactic acid and its derivatives could potentially replace voluminously consumed petroleum that is currently used to make plastic and fine chemicals [8-9]. A process to produce pure lactic acid with a high yield from the abundant and renewable carbohydrates at low energy cost must be developed before a biorefinery platform can be built on the basis of this substrate. Currently lactic acid is produced by glucose fermentation, and the drawbacks for enzyme catalysis reaction are long reaction time and very strict reaction condition [10]. ONDA et al [11-12] reported a reaction to prepare lactic acid by catalytic decomposition of glucose with sodium hydroxide. The reaction could be carried out below 100 ℃. However, in order to obtain lactic acid with a high yield of 57%, the excess amount of sodium hydroxide must be used. Excessive sodium hydroxide leads to a number of problems. It is particularly difficult to separate sodium lactate from sodium hydroxide. JIN et al [13] reported an improvement for this process through adding the hydrogen peroxide to oxidize all organic molecules to formic acid at a yield of 75%.
ZHAO et al [14] suggested that ionic liquid can dissolve glucose and enhance the acid-catalyzed decomposition of glucose. According to their experiment, the ionic liquid [AMIM]Cl showed higher excellent solubility to salts and glucose. In this work, the ionic liquid [OMIM]Cl was used as solvent to enhance the yield of lactic acid for the glucose decomposition reaction.
2 Experimental
Ionic liquids were prepared according to the procedures described in Refs.[15-16]. Glucose (>99%, mass fraction) was used as a test material because it was a primary intermediate compound during the conversion of carbohydrates. 1.0 mol/L standardized solution lactic acid (Alfa Aesar Company, China) was also used for the quantitative analysis of lactic acid. NaOH (99%) and Ca(OH)2 (99%) were selected as alkaline catalysts obtained from China National Medicine Corporation Ltd.
All experiments were carried out in a bath micro- reactor made up of stainless steel with an internal volume of 5 mL. The typical experimental procedures were as follows: the desired amount of glucose, and the desired solid base catalysts with quantitative ionic liquid prepared in advance were put into a batch heated with a temperature programming oven. After a desired reaction time, the reaction time was defined as the time that reactor was kept in the oven. 0.400 g test material was used in all the experiments.
After the reaction was finished, the samples were diluted to about 10 mL with de-ionized water. The solution was extracted and filtered, adding hydrochloride acid to adjust pH<7. The samples were injected in HPLC. HPLC analysis was performed with a Agilent LC-1200 system equipped with a UV detector (214 nm). A C-18 column was used to separate the samples for the analysis of lactic acid, with volume ratio of water to acetonitrile of 95?5 as mobile phase.
The product was characterized by 1H NMR spectra and WATERS LC-MS. The lactic acid yield was defined as the percentage of lactic acid mass to initial glucose mass on the carbon-atom basis.
3 Results and discussion
3.1 Characteristic of ionic liquid in base-catalyzed glucose decomposition reaction
Five kinds of ionic liquids were chosen to evaluate the influences of the ionic liquids. The reactions were carried out at 363 K for 60 min, adding the same amount of glucose (0.400 g), calcium hydroxide (0.150 g), and ionic liquid fixed at 1.000 g.
Table 1 demonstrates that all samples have similar yields of lactic acid. The results indicate that ionic liquids act as solvent and have similar solubility for glucose and calcium hydroxide. For entry 4 and entry 5, ionic liquids have a long alkyl chain which makes them hydrophobic. But they still show good solubility for glucose and calcium hydroxide. Entry 4 and entry 5, have the same alkyl ion, but different anions chloride for entry 4 and bromide for entry 5. According to the yields of lactic acid, the effect of anion ion can be excluded. In ionic liquid system, glucose reacts with calcium hydroxide to form calcium lactate which is easy to dissolve in water. Hydrophobic ionic liquid and calcium hydroxide are facile to separate with calcium lactate by adding water as extracting agent. So in next experiments, C22H43ClN2 was chosen as standard solvent to evaluate the reaction system.
Table 1 Influence of ionic liquids characteristic on yield of lactic acid
3.2 Effect of reaction time on preparation of lactic acid
In order to find the best condition for preparation of lactic acid, three samples were chosen to evaluate the influence of reaction time. The reactions were carried out in a stainless steel bath. The amount of glucose was fixed at 0.400 g, that of calcium hydroxide was 0.150 g and that of the ionic liquid C22H43ClN2 was 1.000 g. Because the melting point for the ionic liquid is about 353 K the samples covered the range from 353 to 373 K. Fig.1 shows that the sample reacted at 353 K has obviously different results compared with results of other samples. The yield of lactic acid is near 5% at first, and as reaction time increases, it does not show a distinct improvement. After 60 min, the yield still preserves at near 5% and keeps at this value for further increment of time. For the experiments at 363 K and 373 K, the lactic acid yields initially increase until 60 min and then decrease for subsequent rise in time. Both of these data show reaction time of 60 min is an advantageous time for obtaining a high yield of lactic acid. The highest yield at 363 K for 60 min is about 55%, which is larger than 45% at 373 K.

Fig.1 Influence of reaction time on yield of lactic acid (Reaction condition: 0.400 g glucose, 1.00 g ionic liquid, 0.150 g calcium hydroxide for every samples)
In order to further confirm the influence of temperature on yield of lactic acid and to find the advantageous condition for lactic acid production, a series of experiments were conducted. In these experiments, the reactions were carried out in the same conditions with different reaction temperatures. To evaluate the influence of temperatures more clearly 333 K was chosen as the lowest point, and 464 K was the highest for this investigation. At the stage of low temperature such as 343 K, calcium hydroxide does not show activity. This may imply that ionic liquids are unable to dissolve both glucose and calcium hydroxide under the solid condition. If the temperature is higher than their melting point, the yield of lactic acid increases rapidly. Fig.2 shows that from 353 to 373K, the yield jumps from 5% to 55%. But the further increment of temperature such as between 373 and 400 K may lead to a drop of the yield to 20% at 400 K. If the temperature is higher than 400 K, the yield does not dramatically decline and keep at 10%. The results demonstrate that both time and temperature have important influences upon the production of lactic acid. Consequently, the highest lactic acid yield of 55% occurs at 363 K for 60 min.

Fig.2 Influence of reaction temperature on yield of lactic acid (Reaction condition: 0.400 g glucose, 60 min, calcium hydroxide as catalyst, [OMIM]Cl as reaction solvent)
The above results have advantages compared with the results in water solution using sodium hydroxide as catalyst with the yield of 41%. As we know, in water/sodium hydroxide reaction system, glucose would decompose to generate formic acid and acetic acid as by-products. But the HPLC results show that formic acid and acetic acid are not observed in ionic liquid reaction system. This is probably due to a low movement ability of both hydroxide anion and lactic acid in a pure ionic liquid solvent system.
3.3 Effect of solid base and ionic liquid for preparation of lactic acid
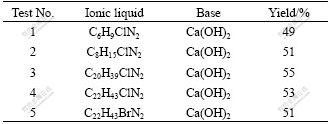
At the beginning, the amount of calcium hydroxide increased from 100% (entry 1) to 270% (entry 4), based on the mole ratio of hydroxide ion to glucose, fixing the mass of C22H43ClN2 at 1.000 g. Table 2 shows that the increment of calcium hydroxide can enhance the yield effectively. Especially, the excessive supply of calcium hydroxide has an obvious effect. The results are in accordance with those using sodium hydroxide in the water system. In the acid condition, glucose is inclined to generate HMF, levulinic acid and formic acid. Lactic acid is an organic acid which is capable of initiating an acid-catalyzed glucose reaction. So, excessive supply of calcium hydroxide may be used to neutralize lactic acid. The effect of ionic liquid supply on the preparation of lactic acid is similar with that of the solid base (calcium hydroxide). In entry 8, a yield of lactic acid (about 100%) is achieved using more ionic liquid as solvent. 1H NMR spectra confirm that only the peaks which belong to lactic acid exist in the spectra.
Table 2 Influence of mole ratio of base to solvent on yield of lactic acid
The technology may good enough to be commercialized with a recycled ionic liquid reaction system. For this reaction, it has the same reaction mechanism with that in water. But the distribution of products is in favor of lactic acid, which may be caused by glucose and calcium hydroxide existing as another form in the solution of ionic liquid.
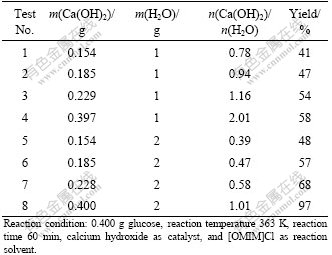
3.4 Effect of different bases on yields of lactic acid
As mentioned previously, a good reaction condition for preparing lactic acid was identified. In this investigation, the catalyst was always set as calcium hydroxide. A series of experiments were carried out to evaluate the influence of different alkaline and alkaline oxides. The results are shown in Table 3. From Table 3, it can be seen that most of the catalysts show activity for the reaction, especially some oxides such as MgO and hydroxides such as Ba(OH)2. In general, saturated solubility of the catalysis is very low in water, which causes them to have weak performance in the production of lactic acid. For example, the yields in Ca(OH)2 and Ba(OH)2 conditions are not more than 5%, and MgO even does not show catalytic effect for the any formation of lactic acid because the saturated solubility of MgO is only 0.84 mg per 100 g water. Generally speaking, it is disadvantageous for catalytic efficiency of solid alkaline oxides and hydroxides compared with homogenous catalysts because homogenous reactions provide a nice contact among reactive reagents. According to the results of these insoluble catalysts (entry 1, 3, 4, 5 and 7), ionic liquids provide a good solubility for these catalysts. In this reactive system, the alkaline-catalyzed reactions in the solution of ionic liquids are homogeneous processes. After the completion of reaction, water is introduced into stainless steel autoclave to make the system heterogeneous. The design is an advanced technology that solves the problem of low catalytic effect of heterogeneous catalysts and the difficulty of separation of homogeneous catalysts.
According to the results in Table 3, both oxides and hydroxides can catalyze the reaction, so the oxygen atom may be the active part of the catalysts. Calcium hydroxide (55.0%) and sodium hydroxide (51.0%) show the highest catalytic efficiency for production of lactic acid because both of them have the strongest basic capacity.
Table 3 Influence of different bases on yield of lactic acid
3.5 Recycle of hydrophobic ionic liquids
As the investigations above mentioned, a series of conditions were explored, but the most important for these reactive systems is the recycle capability of hydrophobic ionic liquids. C22H43ClN2 is evaluated. The result shows that it is available for the production of lactic acid. So it is chosen as solvent for the recycle test. The results are shown in Table 4. Every test was carried out in the same reactive conditions: 0.400 g glucose, 0.150 g calcium hydroxide, and 363 K for 60 min. After reaction, water was used for three times to extract calcium lactate and other products that can solve in water, and hydrochloric acid was used to wash calcium hydroxide that is insoluble in water. The remnant solid is the ionic liquid and is heated at 393 K for 12 h to evaporate water. Table 4 demonstrates that the yield of lactic acid can keep at a stable level, the loss is less than 5% based on mass for every time in this recycle program. After all, ionic liquid essentially has a surfactant structure and a certain water solubility.
Table 4 Effect of recycle of hydrophobic liquids on yield of lactic acid
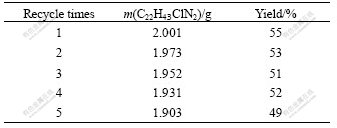
3.6 1H NMR spectra of ionic-liquids comparison
Fig.3 shows 1H NMR spectrum the fresh ionic- liquid. Fig.4 shows 1H NMR spectrum of the products washed by de-ionic water three times. This process was used to extract lactate and glucose which both could solve in water. Because the decomposition products of C22H43ClN2 have a long carbon chain that is unsolvable in water, they can form new peaks in 1H NMR spectrum. Fig.3 and Fig.4 have peaks that belong to ionic liquid chemical shift of 1.130. After reaction, they do not form any new peaks, indicating that the ionic liquid is not involved in the reaction of glucose with base. It is only used as solvent to prompt the reaction and kept as original formation during reaction process.
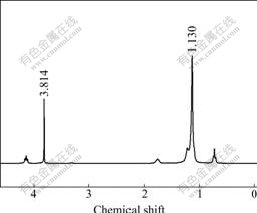
Fig.3 1H NMR spectrum for ionic liquid (C22H43ClN2) as standard sample before reaction
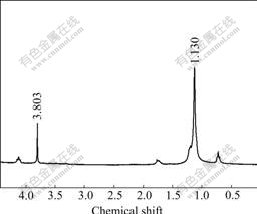
Fig.4 1H NMR spectrum of used ionic liquid (C22H43ClN2) washed by water after reaction completion
4 Conclusions
(1) A new reaction system is found to prepare lactic acid using hydrophobic ionic liquid as solvent. The ionic liquid solvents have good solubility for many kinds of bases, especially some insoluble salts.
(2) The optimal reaction conditions are as follows: 0.400 g calcium hydroxide, 0.400 g glucose in 2.000 g hydrophobic ionic liquid ([OMIM]Cl) reaction temperature 363 K, and reaction time 60 min, and the yield reaches about 100%.
(3) Because calcium hydroxide and ionic liquid are hydrophobic, water can extract lactic acid in the form of calcium lactate from products. After reaction, ionic liquid still keeps as the initiative molecular formation and can be used again.
References
[1] LESHKOV Y R, CHHEDA J N, DUMESIC J A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose [J]. Science, 2006, 312: 1933-1937.
[2] ONDA A, OCHI T, KAJIYOSHI K, YANAGISAWA K. Lactic acid production from glucose over activated hydrotalcites as solid base catalysts in water [J]. Catalysis Communication, 2008, 9: 1050-1053.
[3] BRUIJN J M, TOUWSLAGER F, KIEBOOM A P G, BEKKUM H. Alkaline degradation of monosaccharides. Part Ⅷ: A13C NMR spectroscopic study [J]. Starch, 1987, 39(2): 49-52.
[4] YANG B Y, MONTGOMERY R. Alkaline degradation of glucose: Effect of initial concentration of reactants [J]. Carbohydrates Research, 1996, 280(1): 27-45.
[5] THEADER O, NELSON D A. Aqueous, high-temperature transformation of carbohydrates relatives to utilization of biomass [J]. Advanced Carbohydrates Chemical Biochemistry, 1998, 46: 273-326.
[6] BICKER M, ENDRES S, OTT L, VOGEL H. Catalytical conversion of carbhydrates in subcritical water: A new chemical process for lactic acid production [J]. Journal of Molecular Catalysis, 2005, 239: 151-157.
[7] JIN F, ZHOU Z, ENOMOTO H, MORIYA T, HIGASHIJIMA H. Conversion mechanism of cellulosic biomass to lactic acid in subcritical water and acid-base catalytic effect of subcritical water [J]. Chemistry Letters, 2004, 33: 126-127.
[8] AMASS W, AMASS A, TIGHE B. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies [J]. Polymer International, 1998, 47: 89-144.
[9] CORTRIGHT R D, CASTILLO M S, DUMESIC J A. Conversion of biomass to 1, 2-propanediol by selective catalytic hydrogenation of lactic acid over silica-supported copper [J]. Applied Catalysis B, 2002, 39: 353-359.
[10] LUNT J. Large-scale production, properties and commercial applications of polylactic acid polymers [J]. Polymer Degradation and Stability, 1998, 59: 145-152.
[11] ONDA A, OCHI T, KAJIYOSHI K, K YANAGISAWA K. A new chemical process for catalytic conversion of D-glucose into lactic acid and gluconic acid [J]. Applied Catalysis A: General, 2008, 343: 49-54.
[12] ONDA A, OCHI T, YANAGISAWA K. Selective hydrolysis of cellulose into glucose over solid acid catalysts [J]. Green Chemistry, 2008, 10: 1033-1037.
[13] JIN F M, YUN J, LI G M, KISHITA A, TOHJI K, ENOMOTO H. Hydrothermal conversion of carbohydrate biomass into formic acid [J]. Green Chemistry, 2008, 10: 612-615.
[14] ZHAO H, HOLLADAY J E, BROWN H, ZHANG Z C. Metal chloride in ionic liquid solvents convert sugar to 5-hydroxymethylfurfural [J]. Science, 2007, 316: 1597-1600.
[15] VARMA R S, NAMBOODIRI V V. An expeditious solvent-free route to ionic liquids using microwaves [J]. Chemical Communication, 2001: 634-644.
[16] BONHOTE P, DIAS A, PAPAGEORGIOU N, KALYANASUNDARAM K, GRATZEL M. Hydrophobic, high conductive ambient-temperature molten salts [J]. Inorganic Chemistry, 1996, 35: 1168-1178.
Foundation item: Project(2006BAE02B05) supported by the Key Projects in the National Science and Technology Pillar Program During the 11th Five-year Plan Period; Project(2005CB221406) supported by the National Basic Research Program of China
Received date: 2009-03-18; Accepted date: 2009-06-17
Corresponding author: ZHOU Xiao-ping, Professor; Tel: +86-731-88821017; E-mail: hgx2002@hnu.cn
(Edited by YANG You-ping)