J. Cent. South Univ. Technol. (2007)06-0763-05
DOI: 10.1007/s11771-007-0145-6
Depressing effect of phenoxyl acetic acids on flotation of minerals containing Ca2+/Mg2+ gangues
ZHANG Jian-feng(张剑锋)1, HU Yue-hua(胡岳华)2, WANG Dian-zuo(王淀佐)2, XU Jin(徐 竞)2
(1. State Key Laboratory Base of Novel Functional Materials and Preparation Science, Faculty of Materials Science and Chemical Engineering, Ningbo University, Ningbo 315211, China;
2. School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract:Phenoxyl acetic acids were applied to determine their depressing effect on minerals containing Ca2+/Mg2+ gangues. Calcite, mixture of calcite and fluorite, and nickel ore were used in the flotation. And the depression mechanism was studied by the determination of contact angle, zeta potential, adsorptive capacity of collector, and IR analysis as well. It is found that 0.1 mmol/L of phenoxyl acetic acid derived from pyrogallol or gallic acid exhibits strong depressing ability on calcite in almost zero yields at pH value of 9.8, and calcite can be depressed in the flotation of calcite/fluorite mixture for approximate 87% yield of fluorite. The flotation result of practical nickel ore containing serpentine indicates that these two depressants may also show better depression performance to serpentine than traditional depressants such as sodium fluosilicate and carboxylmethyl cellulose. Analysis for the depression mechanism reveals that there exists strong chemical interaction between the depressants and minerals.
Key words: flotation; depressing effect; depressant; phenoxyl acetic acid; calcite; serpentine
1 Introduction
Phenol and polyphenols, such as diphenol, pyrogallol and gallic acid, are facile organic chemicals from nature or synthesis. There are many practical applications for phenol and polyphenols because of their multiple reactive characteristics of characterized benzene-ring and phenolic hydroxyl group[1-2]. Phenolic hydroxyl group exhibits classical Williamson reaction with mono-chloroacetic acid, which can generate carboxylic ether with greater hydrophilicity and water solubility that might be used as flotation depressants. But it is difficult to carry out Williamson reaction of polyphenols because of their easy oxidation in alkaline solution and open air condition. Only several reports about Williamson reaction of diphenols were presented in Refs.[3-6], while very few reports of pyrogallol and gallic acid can be available. ZHANG et al[7-9] synthesized phenol and polyphenol derivatives. In this work, the depressing effect of phenoxyl acetic acids on the flotation of minerals with Ca2+/Mg2+ gangues was investigated.
2 Experimental 2.1 Chemicals and materials
Phenol, polyphenols, and sodium monochloro-acetic acid were analytical grade. Other reagents used were of chemical grade. Tributylethylammonium ethosulfate was kindly contributed by Dr. LI from Central South University, Changsha, China. Calcite, fluorite, and nickel ore were obtained from Beijing Ore Powder Plant, Dongfang Fluorite Mine and Jinchuan Nickel Mine, respectively, and were ground in a porcelain mill and screened in distilled water with the size from 37 to 70 μm. The purities of calcite and fluorite were 99.1% and 98.5%, respectively. The mass fractions of copper and nickel in nickel ore were 0.82% and 1.52%, respectively.
2.2 Preparation of phenoxyl acetic acid
According to Ref.[5], 0.05 mol of phenol or polyphenol material, 0.002 mol of tributylethy- lammonium ethosulfate, and excessive 20% (molar fraction) sodium mono-chloroacetic acid were poured into a three-necked flask with 120 mL chloroform solvent at 0 ℃. Proper amount of 10 mol/L pre-cooled sodium hydroxide was slowly dripped into the flask under constant stirring. The reaction flask was equipped with machinery agitation apparatus and reflex condenser. At the top of the condenser a simple self-sealing device was tied to keep the system from atmosphere. The mixture was heated to reflux for 1 h and then cooled, and hydrochloric acid was added till the point of Congo red changing to blue. The solution was then cooled forcomplete crystallization and filtered to obtain phenoxyl acetic acid. Phenoxyl acetic acid was purified by recrystallization in water and dried in vacuum.
2.3 Flotation test
Flotation tests were conducted in 50 mL laboratory microflotation cell with 2.0 g of materials at a stirring rate of 1 650 r/min by a procedure as follows: adjusting pH value with NaOH and HCl to proper value, stirring for 2 min after addition of the depressant, 2 min after addition of collector of sodium oleate, and 3 min for flotation.
2.4 IR spectra test
IR spectra were recorded on Perkin Elmer Corporation Instrument System 2000 FT-IR by utilizing the KBr pellet technique.
3 Results and discussion 3.1 Depression effect on calcite
The structures of the synthesized phenol acetic acids are shown in Fig.1, indicating that phenol acetic acids can easily dissolve in aqueous solution, and have the potentiality to be used as depressant of flotation for oxidized minerals, especially for calcareous and magnesium minerals. Calcite, a common gangue in oxidized minerals, was used as a sample to determine the flotation effect of phenoxyl acetic acid. The results of flotation are shown in Fig.2. It can be seen that all of the seven phenoxyl acetic acids exhibit depressing effect on calcite, and the depressants (f) and (g) exhibit intensive depression. Calcite can be completely depressed at pH value of 9.8 in the presence of depressant (f) or (g) with concentration of 0.1 mmol/L. Further study (see Fig.3) shows that calcite can be depressed completely in 0.025-0.075 mmol/L depressants (g) and (f) respectively, which agrees that depressant (g) is a bit more efficient than depressant (f).
3.2 Effect on separation of gangues from mixtures
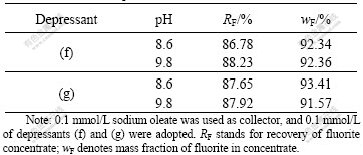
The separation of fluorite from calcareous minerals is a very important but difficult process in flotation. Calcite has similar surface property with fluorite, which results in difficulty in separating fluorite from calcite[10-13]. The results of separation for mixture of calcite and fluorite are listed in Table 1. It can be seen that calcite and fluorite can be separated by depressants (f) and (g), and that there is no apparent difference in depressing effect between depressants (f) and (g).
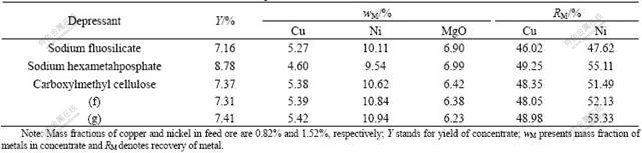
Jinchuan Nickel Mine owns the largest nickel- containing ore deposit of copper-nickel sulfide type in China, and it has the second reserves of serpentine nickel sulfide in the world. But it is very difficult to separate the useful nickel mineral from the gangue because of the complex composition, inhomogeneous particle size, and magnesium-containing gangue[14-16]. Serpentine, the main gangue of Jinchuan nickel ore, is mainly composed of magnesium silicate that can cause great interference of the flotation process for its severe argillization and lead to low grade concentrate with much amount of MgO. Magnesium silicate has some common characteristic chemical structures with calcium carbonate. Both depressants of (f) and (g), which show strong depression effect on calcite, were tested for the flotation of Jinchuan nickel ore (see Table 2), and some classical depressants were also applied for a comparison. The results show that the mass fraction of MgO in the concentrate is lower than 6.4%, that of nickel higher than 10.8%, and the recoveries of Cu and Ni are over 48% and 52%, respectively, when depressants (f) and (g) are used. It also shows that depressants (f) and (g) are promising depressants for serpentine with better flotation results than the classical depressants.
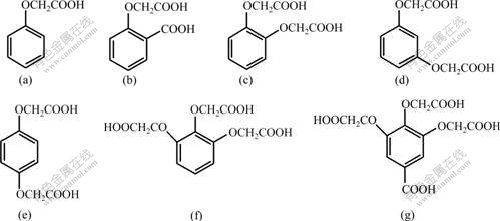
Fig.1 Structures of synthesized phenoxyl acetic acids
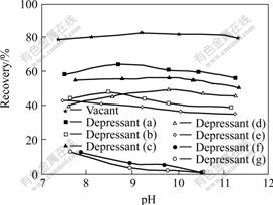
Fig.2 Recoveries of calcite as function of pH values in presence of phenoxyl acetic acids
(Concentrations of collector and reagent are both of 0.1 mmol/L)
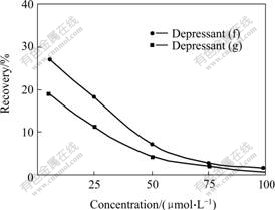
Fig.3 Recovery of calcite as function of concentration of depressants (f) and (g) at pH=9.8 and sodium oleate concentration of 0.1 mmol/L
Table 1 Results of separation for mixture of calcite and fluorite
3.3 Mechanism of depressants to calcite
Further studies were conducted to determine the depressing mechanism of depressants (f) and (g) on calcite. From the result of contact angle variation (see Fig.4), it can be seen that phenoxyl acetic acids (f) and (g) can decrease the contact angle of calcite, and they can be adsorbed on the calcite surface, resulting in a lower flotability of calcite. It can also be seen that depressant (g) can reduce the contact angle of calcite a bit more largely than depressant (f), which means that depressant (g) is slightly more hydrophilic than depressant (f). This is quite consistent with the chemical structures of depressants (f) and (g) because there are only three carboxymethyl groups in molecule (f), but another additional carboxyl group in molecule (g). The result of zeta potential determination (see Fig. 5) also shows that the IEP (isoelectric point) of calcite is at pH=9.5, depressants (f) and (g) change the zeta potential of calcite from positive to negative at pH
Fig.6 shows the adsorption density of oleate on calcite surface in the presence of depressants (f) and (g). With the increase of concentration of depressant, the adsorption of oleate on calcite surface decreases. It can be deduced that depressants (f) and (g) can depress the mineral by a competitive adsorption on the mineral surface against the collector. It is supposed that depressants (f) and (g) are adsorbed on calcite surface prior to sodium oleate and relieve the adsorbed collector from mineral surface by desorption. Both the polar groups for oleate and depressants (f) or (g) are carboxyl groups, but there exist some differences between them. The intensive interaction of phenoxyl acetic acid on calcite surface should mostly result from the strong electrophilic effect of oxygen atom close to carboxyl group; nevertheless there is no such electronic effect for carboxyl group of oleate. Therefore, the carboxyl groups in depressants (f) and (g) may exhibit greater polarity than that in oleate. It means that the former can form
Table 2 Results of separation for nickel ore from Jinchuan Nickel Mine
more intensive ionic bond with Ca2+, and then depression effect is achieved. IR spectra analysis approves this viewpoint (see Fig.7). On the spectra of calcite adsorbed with depressants (f) and (g), a weak carbonyl peak emerges at 1 752 and 1 765 cm-1, and also the vibration peaks specific aromatic C-H of are defined at 3 031 and 3 028 cm-1, respectively.
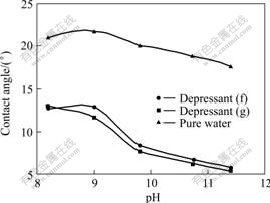
Fig.4 Effect of depressant on contact angle of calcite (concentrations of depressants (f) and (g) and collector are all of 0.1 mmol/L)
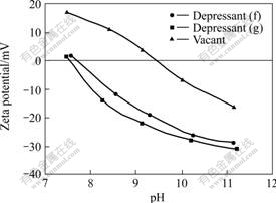
Fig.5 Effect of depressant on zeta potential of calcite (concentrations of depressants (f) and (g) are all 0.1 mmol/L)
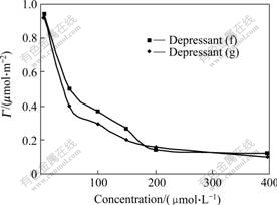
Fig.6 Adsorptive density of oleate on calcite surface as function of depressant concentration at sodium oleate concentration of 0.1 mmol/L
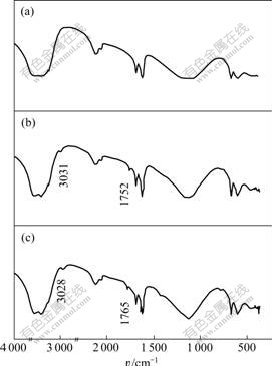
Fig.7 IR spectrum comparison of calcite and calcite absorbed by depressants
(a) Calcite; (b) Calcite+depressant (f); (c) Calcite+depressant (g)
4 Conclusions
1) Phenoxyl acetic acids derived from pyrogallol and gallic acid exhibit strong depressing effect on calcite.
2) Mixtures of calcite and fluorite can be separated by using two phenoxyl acetic acids for the selective depression on calcite, and the two phenoxyl acetic acids show better performance than traditional depressants on serpentine in Jinchuan nickel ore.
3) There exists strong chemical interaction between phenoxyl acetic acids and mineral surface, which leads to the depressing effect on the mineral.
4) Phenoxyl acetic acids are promising depressants for flotation of minerals containing Ca2+/Mg2+ gangues.
References
[1] ARKHIPOV V V, SMIRNOV M N, KHILYA V P. Chemistry of modified flavonoids. 19. Synthesis of phenoxyl analogs of isoflavone[J]. Chem Heterocycl Compd, 1997, 33(5): 515-519.
[2] MUKAI K, NISHIMURA M, NAGANO A, et al. Kinetic study of the reaction of vitamin C derivatives with tocopheroxyl (vitamin E radical) and substituted phenoxyl radicals in solution[J]. Biochim Biophys Acta, 1989, 993(2/3): 168-173.
[3] MIRCI L E. Production and purification of phenylenedioxyacetic acid: Romania, RO 100132[P]. 1990-06-27.
[4] SUGURO Y, MSTASUMOTO M. Preparation of 1, 3-pheny- lenedioxydiacetic acid as monomer: JP, 91052[P]. 1992-03-24.
[5] SUGURO Y, MSTASUMOTO M. Preparation of 1,3-pheny- lenedioxydiacetic acid with low discoloration: JP, 173764[P]. 1992- 06-22.
[6] SUGURO Y, MSTASUMOTO M. Preparation of 1,3- phenylenedioxydiacetic acid with low discoloration: JP, 173765[P]. 1992-06-22.
[7] ZHANG Jian-feng, HU Yue-hua, WANG Dian-zuo. Preparation of phenoxyl acetic acid and its depression on calcite[J]. J Cent South Univ Technol, 2001, 32(2): 146-149. (in Chinese)
[8] ZHANG Jian-feng, HU Yue-hua, WANG Dian-Zuo. Phase transfer catalyzed synthesis and flotation performance of biphenoxyl bi(acetic acid)[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 707-711. (in Chinese)
[9] ZHANG Jian-feng, LI Xiao-ru, LIU Jia-jia, et al. Synthesis of 1,2,3-phenyltrioxyl tri(acetic acid) by phase-transfer catalysis[J]. J Cent South Univ Technol, 2001, 32(5): 491-493. (in Chinese)
[10] ZHANG Ya-hui, LU Yin-zhi, XU Shi. Reaction mechanism of (1, 2, 3)-trihydroxybenzene in separation of fluorite from calcite by flotation[J]. Nonferrous Metals, 1991, 43(3): 22-27, 21. (in Chinese)
[11] SHI Wei, HUANG Guo-zhi. Solubility and flotation separation of calcite and fluorite[J]. Non-Metallic Mines, 2000, 23(4): 11-12. (in Chinese)
[12] LI Ye, LIU Qi, XU Shi. Adsorption properties and interaction mechanism of starch-type polysaccharides onto fluorite and calcite[J]. Nonferrous Metals, 1996, 48(1): 26-30, 15. (in Chinese)
[13] LI Ye, XU Shi, LIU Qi. Application and interaction mechanism of polymeric carbohydrate in oxides and salt-type minerals[J]. Journal of Wuhan University of Technology: Materials Science, 1996, 11(4): 43-47. (in Chinese)
[14] GAO Yu-de, HU Chun-hui, DENG Li-hong, et al. Study on the reduction of MgO content in nickel concentrate in Jinchuan[J]. Journal of Guangdong Non-ferrous Metals, 2000, 10(2): 96-99. (in Chinese)
[15] FENG Qi-ming, ZHANG Guo-fan, LU Yi-ping. The effects of serpentine on blucite flotation and present status for studies of its depressant[J]. Conservation and Utilization of Mineral Resources, 1997(5): 19-22. (in Chinese)
[16] ZHANG Guo-fan, LU Yi-ping, FENG Qi-ming. A study on reducing the content of MgO in nickel concentrate with depressant EP[J]. Conservation and Utilization of Mineral Resources, 1999(3): 28-31. (in Chinese)
(Edited by CHEN Wei-ping)
Foundation item: Project(Year 2005) supported by the Plan of High Learning Young Teacher of Zhejiang Province, China; Project(2005A620015) supported by the Ningbo Youth Science Fund, China; Project(2004745) supported by the Ningbo University for PhD
Received date: 2007-03-02; Accepted date: 2007-04-18
Corresponding author: ZHANG Jian-feng, PhD; Tel: +86-574-87600745; E-mail: zjf@nbu.edu.cn