Review of studies on corrosion of magnesium alloys
来源期刊:中国有色金属学报(英文版)2006年第z1期
论文作者:曾荣昌 张津 黄伟九 W. DIETZEL K. U. KAINER C. BLAWERT 柯伟
文章页码:763 - 771
Key words:magnesium alloy; intermetallic phase; corrosion; fatigue; weld; relative humidity
Abstract: This review provided some recent progress of the research on corrosion mechanisms of magnesium and its alloys and a basis for follow-on research. Galvanic corrosion, pitting corrosion, intergranular corrosion (IGC), filiform corrosion, crevice corrosion, stress corrosion cracking (SCC), and corrosion fatigue (CF) were discussed. The influence of metallurgical factors such as alloying elements, microstructure and secondary phases, processing factors such as heat treatment and weld, and environmental factors including temperature, relative humidity, solution pH values and concentration on corrosion were discussed. In particular, a mechanism of pitting corrosion caused by AlMn particles was proposed. The corrosion properties of AZ91D weld material were investigated.

ZENG Rong-chang(曾荣昌)1, ZHANG jin(张 津)1, HUANG Wei-jiu(黄伟九)1, W. DIETZEL2,
K. U. KAINER2, C. BLAWERT2, KE Wei(柯 伟)3
1. School of Material Science and Engineering,
Chongqing Institute of Technology, Chongqing 400050, China;
2. GKSS-Forschungszentrum Geesthacht GmbH, Geesthacht 21502, Germany;
3. Environment Corrosion center, Institute of Metals Research, Shenyang 110016, China
Received 10 April 2006; accepted 25 April 2006
Abstract This review provided some recent progress of the research on corrosion mechanisms of magnesium and its alloys and a basis for follow-on research. Galvanic corrosion, pitting corrosion, intergranular corrosion (IGC), filiform corrosion, crevice corrosion, stress corrosion cracking (SCC), and corrosion fatigue (CF) were discussed. The influence of metallurgical factors such as alloying elements, microstructure and secondary phases, processing factors such as heat treatment and weld, and environmental factors including temperature, relative humidity, solution pH values and concentration on corrosion were discussed. In particular, a mechanism of pitting corrosion caused by AlMn particles was proposed. The corrosion properties of AZ91D weld material were investigated.
Key words: magnesium alloy; intermetallic phase; corrosion; fatigue; weld; relative humidity
1 Introduction
The standard electrode potential of magnesium ![]() is -2.37 V, but in 3% sodium chloride the electrode potential is -1.63 V(vs SCE). Therefore magnesium is often used as a sacrificial anode. On the other hand, magnesium offers a high potential for use as a lightweight structural material in transport applications such as automotive and motorbike. Since these may be subjected to serious environments, their corrosion behavior is a major concern. The reason for the poor corrosion resistance of magnesium and its alloys lies on two aspects: 1) the oxide films forming on surface are not perfect and protective; 2) galvanic or bimetallic corrosion can be caused by impurities, secondary phases such as Mg17Al12 [1-4], AlMn [5], Al8Mn5 [6], Mg12Nd [7], Mg2Pb [3] etc, even when connected with notably iron, nickel and copper.
is -2.37 V, but in 3% sodium chloride the electrode potential is -1.63 V(vs SCE). Therefore magnesium is often used as a sacrificial anode. On the other hand, magnesium offers a high potential for use as a lightweight structural material in transport applications such as automotive and motorbike. Since these may be subjected to serious environments, their corrosion behavior is a major concern. The reason for the poor corrosion resistance of magnesium and its alloys lies on two aspects: 1) the oxide films forming on surface are not perfect and protective; 2) galvanic or bimetallic corrosion can be caused by impurities, secondary phases such as Mg17Al12 [1-4], AlMn [5], Al8Mn5 [6], Mg12Nd [7], Mg2Pb [3] etc, even when connected with notably iron, nickel and copper.
MAKER et al [1-4, 8-10] have reviewed the corrosion of magnesium alloys. These prior reviews have summarized the corrosion mechanisms of magnesium and its alloys and the progress made in each stage.
This review provided some recent progress and presented some important issues from a scientific point of view in the field of corrosion of magnesium alloys.
2 Types of corrosion
2.1 Galvanic corrosion
Magnesium alloys are susceptible to galvanic corrosion due to excessive levels of heavy metal or flux contamination, and to poor design and assembly practices. If these metals such as iron, nickel and copper, have a low hydrogen overvoltage, they can serve as efficient cathodes, consequently causing severe galvanic corrosion. Metals that combine an active corrosion potential with high hydrogen overpotential such as Al, Zn, Cd and Sn, are much less damaging. The galvanic corrosion consisting of substrate and inner secondary phase is macroscopically observed as overall corrosion. The galvanic corrosion behaviour and laws of AZ91D, AM50 and AM60 cast magnesium alloys coupled with A3 steel, 316L stainless steel, H62 brass and LY12 aluminum alloy undergone atmospheric exposure were investigated by TONG et al [11]. The results show that the magnesium alloys act as anode and that their corrosion rates increase when they are coupled with the above mentioned four metals. The atmospheric galvanic effect of magnesium alloys coupled with A3 steel is the largest, while that of the magnesium alloys/LY12 aluminum alloy couple is the lowest. And there is the following ranking of the atmospheric galvanic effects of different magnesium alloys: AZ91D>AM50 >AM60.
High purity magnesium alloys cannot avoid galvanic corrosion if they are coupled with other metals. By proper material selection, proper design and selective use of coatings and insulation materials the risk for galvanic corrosion ban be significantly reduced. For example, fasteners made of aluminum of 6000 series reduce galvanic corrosion of magnesium to very low levels in salt spray tests [12].
2.2 Pitting corrosionMagnesium is a naturally passive metal. Pitting corrosion will occur at free corrosion potential of magnesium, when exposed to chloride ions in a non-oxidizing medium [2]. It is generally observed that corrosion pits initiate at flaws adjacent to a fraction of the secondary phase particles such as Mg17Al12, AlMn as a result of the breakdown of passivity [2, 5]. This is followed by the formation of an electrolytic cell in which the secondary phase particles are the cathode of the type AlMn, AlMnFe, Mg17Al12, Mg2Cu, and the surrounding Mg matrix is the anode. For example, the as-extruded magnesium alloy AM60 was immersed in natural 3.5% NaCl solution, and the corrosion pits occurred on the surrounding of AlMn particles (Fig.1).

Fig.1 Pitting morphology of extruded AM60 in 3.5%NaCl aqueous solution [5]
A model of pitting corrosion mechanism of as-extruded AM60 is given in Fig.2 [5].
1) Firstly, the alloy has a protective oxide film in air. The potential of MgO is +1 V.
2) When it is immersed in a sodium chloride aqueous solution, Cl- ions will absorb on the a areas bordering on AlMn particles.
3) If the breakdown potential of the oxide film reaches its free corrosion potential (φcorr=-1.53 V for AM60), then the a-matrix as an anode, compared to
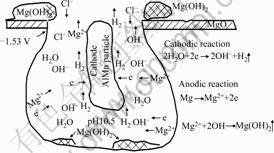
Fig.2 Scheme of pitting corrosion mechanism for magnesium alloy AM60 [5]
AlMn particles, starts to dissolve, and a corrosion nucleus may form nearby an AlMn particle.
4) The nucleus develops a corrosion pit, this may result in Mg (OH)2 formation and hydrogen evolution according to the chemical reactions:
Anodic reaction:
Mg→Mg2++2e (1)
cathodic reaction:
2H2O+2e→2 H2↑+2OH- (2)
Total reaction:
Mg2++2H2O=Mg(OH) 2+2H2↑ (3)
5) At the end, an occlusion cell or a hemi-spherical corrosion pit will be formed with the corrosion proceeding. The pH value will finally reach and keep at 10.4-10.5. Mg hydroxide precipitates on the bottoms of pits and surfaces of samples.
2.3 Intergranular corrosion
Intergranular corrosion (IGC) occurs at the grain boundaries due to the precipitation of secondary phase. The grain boundaries are always the preferred sites at which precipitation and segregation in alloys occur. It is generally regarded that alloys with intermetallic phases or compounds are highly susceptible to intergranular corrosion. It is argued whether magnesium alloys suffer intergranular corrosion or not. MAKER et al [1] insisted that true intergranular corrosion did not occur in Mg alloys. The reason is that the phases at the grain boundaries are almost cathodic to the grains. Corrosion tends to be concentrated in the area adjacent to the grain boundary until eventually the grain may be undercut and fall out [1, 2]. Recent studies, however, show that intergranular corrosion can occur on magnesium alloys. VALENTE [13] noticed that the grain boundaries suffered preferred attack, and intergranular corrosion occurred for WE43 in 3.5% NaCl aqueous solution. GHALI et al [9] also pointed out that in the early stages of immersion, a localized attack of Mg and its alloy can be formed at the grain boundaries at the interface of cathodic precipitates in mild corrosive media and that this can be considered as intergranular (intercrystalline) corrosion. For example, IGC of AE81 can be seen, because the grain bodies with a low Al concentration corrode at a faster rate than that in the Al-rich regions. intergranular corrosion occurred after immersing aged AZ80 in 3.5% NaCl solution for 1 h (Fig.3). Corrosion attacks along the grain boundaries and forms deep and narrow paths.
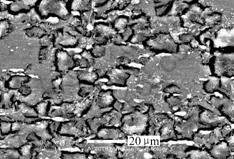
Fig.3 Intergranular corrosion morphology of AZ80-T5 in 3.5%NaCl aqueous solution after 1 h
Ageing results in a decrease of the average aluminium concentration in α matrix. The lower aluminium concentration of aged AZ80 in matrix α formed a less protective oxide film on the surface, and led to a breakdown potential. Therefore, the attack in aged AZ80 easily commenced at α matrix next to β phase in aged AZ80 alloy. It could indeed demonstrate a reduction of aluminium content in the areas rich in aluminium along the grain boundaries. It has been reported that a decrease in aluminium could lead to an increased corrosion rate in α matrix [3]. It is expected that the decreasing average aluminium concentration in α matrix after ageing will result in a decrease of the corrosion resistance in α matrix of as-extruded AZ80.
2.4 Filiform corrosion
Filiform corrosion often occurs on the metals surface such as steel, Al alloys and Mg alloys. It is caused by active galvanic cells across the metal surface. Its head is anodic, whereas the tail is cathodic. It is typically associated with metal surfaces having an applied protective coating. It does not occur on bare pure Mg [8]. LUNDER et al [14] studied the corrosion of AZ91; the earlier stage of corrosion for magnesium alloy AZ91 has a characteristic of pitting and filiform corrosion. Filiform corrosion initiates corrosion pits. DEXTER [15] suggested that the filiform corrosion of magnesium is driven by oxygen concentration between the head and tail, and he proposed a model of filiform corrosion. But this model is in contradiction with the theory that magnesium corrosion is relatively insensitive to oxygen concentration differences.
2.5 Crevice corrosion
It is reported that crevice corrosion does not occur with the magnesium alloys [1, 2]. Though a form of attack that occurs at narrow gaps (“crevice”) appears similar to crevice corrosion, it is not true for crevice corrosion. Because the corrosion observed is caused by the retention of moisture in the crevice which, being unable to evaporate, promotes the corrosion of metal in the narrow recess over extended periods. Filiform corrosion, however, is a special type of crevice corrosion. Now that filiform corrosion can occur with the magnesium alloys, then it is deduced that crevice corrosion may be possible to occur with magnesium alloys. GHALI et al [9] also suggested that crevice corrosion could be initiated due to the hydrolysis reaction in case of Mg and Mg alloys, at least in certain conditions where it is believed that oxygen does not play a major role in the corrosion mechanism. The formation of Mg hydroxide should influence the properties of the interface between the Mg and the solution in the crevice.
2.6 Stress corrosion cracking
Stress corrosion cracking (SCC) is an extremely dangerous type of corrosion damage in engineering service of equipment, vessels etc. The majority of the SCC literature has focused on extruded or rolled Mg alloys [16]. Die-cast alloys are more susceptible to SCC than rapidly solidification (RS) and semi-solid cast alloys [17].
SCC of magnesium alloys can occur in moist air, high purity water, NaCl+K2CrO4 solution, NaBr, Na2SO4, NaCl, NaNO3, Na2CO3, H2SO4, KF, KCl, NaI, MgCO3, NaOH, H2SO4, HNO3, and hydrogen chloride solutions [16]. Alloys containing Al are thought to be particularly susceptible to SCC in air, distilled water and chloride-containing solutions.
SCC in Mg alloys has been generally attributed to one of two groups of mechanisms: continuous crack propagation by anodic dissolution at the crack tip or discontinuous crack propagation by a series of mechanical fractures at the crack tip [17], i.e., there are two types of models for SCC: the dissolution models and the brittle fracture models. The former includes a preferential attack model, film rupture model, tunneling theory etc; the later involves cleavage processes and hydrogen embrittlement (HE) theory.
Based on the fracture morphology of samples, there exist two kinds of SCC: Transgranular SCC (TGSCC) and intergranular SCC (IGSCC). TGSCC is a major mode of SCC. Most Mg alloys have HCP crystal structures, which are susceptible to cleavage due to the less slip systems available. For example, pure Mg suffers TGSCC, and Mg alloys suffer TGSCC in distilled water. And, TGSCC in Mg alloys is related to hydrogen. Experimental evidence supporting a HE mechanism includes[16]: 1) SCC initiation and propagation are companied by hydrogen evolution; 2) Immersion in a cracking solution before stress is applied produces a fracture similar to a SCC fracture; 3) The effect of pre-immersion in a cracking solution is reversed by vacuum annealing or exposure to room-temperature air; 4) Testing in gaseous hydrogen results in the same crack characteristics as those produced in aqueous solution tests; 5) SCC occurs at crack velocities at which only adsorbed hydrogen should be present at the crack tip.
SONG et al [18] inferred that AZ31 sheet was susceptible to SCC in distilled water, ASTM1387 solution, 0.01 mol/L and 0.1 mol/L NaCl solutions. The role of anodic dissolution is to produce surface defects that promote hydrogen production and entry into the material; hydrogen can reduce the cohesive strength of magnesium alloys, hence resulting in hydrogen embrittlement.
The magnesium hydride theory is proposed due to observations of an approximately 1 μm thick brittle MgH2 film on a magnesium SCC fracture surface. IGSCC is not the major fracture mode in magnesium. HE may not always be the primary mechanism for SCC crack growth.
TGSCC is not related to precipitates of any kind. IGSCC has been related to localized galvanic attack of the matrix when coupled with cathodic Mg17Al12 grain-boundary precipitates.
2.7 Corrosion fatigue
A collection of fatigue data of Mg alloys has been gained over the past years [19]. Most of the fatigue data are concerned with fatigue life. There is an endurance limit for magnesium alloys in air. Fatigue strength is improved as the grain size decreases. The reverse was found to be the case for the fatigue crack propagation resistance [20]. Many experimental results showed a significant reduction in fatigue strength or fatigue life in sodium chloride solutions, even in tape water or distilled water [19]. For instance, the corrosion fatigue resistance of magnesium alloys AZ91E-T6 was significantly reduced in 3.5% salt water relative to that in air [21]. And a corrosive medium can more remarkably shorten the fatigue life of extruded magnesium alloys compared with die-cast magnesium alloys [20].
Concerning fatigue testing, especially the test frequency is a very important parameter to be considered in corrosion fatigue, as the corrosion fatigue becomes much more pronounced at lower frequency values in aqueous solution [9]. It is generally assumed that frequency has no effect on fatigue life of metal in air. This is, however, not true for magnesium alloys. For example, the lower the frequency (1-10 Hz), the shorter was the fatigue life of as-extruded AM60 in air [22].
Fatigue cracking of AZ80 initiated at inclusions on the surface or subsurface of samples in air, while it emanated at corrosion pits, which induced microscopic cracks in corrosive solutions. The corrosion pits initiated in the α-Mg matrix on the verge of β particles [23]. Stress-assisted dissolution (SAD) was the mechanism of corrosion fatigue for extruded magnesium alloy AZ80.
The fractography of extruded AM60 showed that fatigue crack initiation was related to AlMn particles. Fatigue cracks emanated at AlMn particles in air, and the corrosion pits bordering upon the AlMn particles in aqueous solutions [22].
3 Influencing factors of corrosion behavior
3.1 Metallurgical influence
3.1.1 Alloying elements
The major alloying elements are Al, Zn, Mn and so on. Fe, Co, Ni and Cu are detrimental for the corrosion of magnesium alloys. Corrosion resistance improves with the Al content [3, 6]. For example, the corrosion rate of AZ91, AZ61 and AZ31 in 5%NaCl solution increased with the decrease of Al content [6]. For AE42, ZAC8506 and AZ91D [24], the corrosion rate decreased in the sequence as: AE42>ZAC8506>AZ91D.
The composition of segregation in the microstructure has an important influence on the corrosion behavior. For example, salt fog corrosion tests for Mn-containing Mg-Al magnesium alloys such as AM50 and AM20, showed corrosion that pits initiated at low Al areas, the matrix was attacked in the form of fissures. The fissures started from pitting locations and usually stopped in front of areas of high Al segregation [6]. The continuous high Al segregation seemed to contribute much more in stopping the propagation of corrosion fissures than the discontinuous, more or less isolated, β-Mg17Al12 particles [6].
Mn can improve the corrosion resistance of magnesium alloys; but this is not always the case. The corrosion rate of magnesium alloys is related to iron content and Fe/Mn ratio. Binary Al-Mn phase with lower Al/Mn ratio has higher cathode potential. Therefore, the corrosion rate increases when Mn is added into Mg-Al magnesium alloys to form Al-Mn and intermetallic phase Al-Mn-Fe [6].
Zr can stabilize the magnesium matrix phase and reduce its corrosion rate. The beneficial effect of Zr cannot be extended to an alloy with too much Zr. The excess addition of Zr can lead to precipitation of Zr in the matrix, which is detrimental to the corrosion [4].
In addition, rare earth improves also the corrosion resistance of magnesium alloys, but is affected by medium and pH value [25].
The influence of copper content on microstructure and corrosion resistance of AZ91 based secondary Mg alloys was reported [26]. Copper addition to the magnesium alloy AZ91D results in grain refining and the formation of additional Mg-Al-Cu-Zn phases. With increasing copper content in the intermetallics, the free corrosion potential will shift to more noble values causing larger problems with local galvanic corrosion. Up to a certain amount, the β phase can be able to embed copper-rich intermetallics, thus preventing direct contact between the Mg matrix and the copper-rich intermetallics.
The susceptibility of SCC increases as the Al content increases from 1% to 8% [16]. The addition of Zn increases the SCC susceptibility. There are conflicted reports on the influences of other elements such as Mn, nd, Zr, Cd, Ce, Sn, etc[17].
3.1.2 Microstructure and secondary phases
The β phase is cathodic with respect to the matrix. It plays a dual role, depending on its volume fraction (f=Vβ/Vα) in the microstructure. It can be used not only as a corrosion barrier [27, 28], and a cathode that causes galvanic corrosion [28]. If f is lower, β phase acts as a cathode that can accelerate the general corrosion of the α-Mg matrix; if f is higher, the β phase can be a barrier inhibiting general corrosion [27]. LUNDER et al [29] studied the role of β phase in the corrosion of AZ91, it is suggested that the β phase has the good properties of the two metals (its corrosion resistance is similar to Mg in alkaline solution and Al in neutral solution); moreover, the corrosion resistance is better than that of Mg and Al in alkaline solution. KO et al [30] investigated the effects of β phase (Mg17Al12 ) on the corrosion behavior of AZ91D, its free corrosion potential increased from -1.648 to -1.585 V (vs SCE),corrosion pit depth of the specimen after potentiaostatic test decreased from 590 to 240 μm,the corrosion rate decreased from 1.73 to 0.31 mm/a, with increasing the volume fraction of β phase from 0 to 10.3%.
AlMn particles are commonly observed in the microstructure of Mg-Al alloys. Table 1 shows that the corrosion potential of AlMn is higher than that of Mg17Al12. Thus, AlMn is more detrimental than Mg17Al12. For example, a corrosion pit of as-extruded AM60 in 3.5% NaCl solution is caused by AlMn particles, rather than β phase (Fig.1). Finally, the XPS analyses revealed that the corrosion layer of MnAl was mainly constituted of an aluminum hydroxide [31].
In addition to the β and AlMn phases, the most potential cathodes in Mg-Al alloys are iron-rich phases, in particular the FeAl phase [3] (table 2). It is one of the most detrimental cathodic phases present in Mg-Al alloys on the basis of its potential and its low hydrogen overvoltage.
Table 1 Corrosion potential of pure phases after 3 h immersion in ASTM D1384 water (initial pH= 8.3) [31]
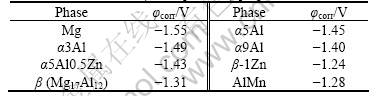
Table 2 typical corrosion potential values for Mg and common Mg second phases (After 2 h) in deaerated 5 % NaCl solution saturated with Mg (OH) 2 (pH = 10.5) [3]

Mg2Pb facilitates pitting and leads to a negative difference effect [3].
Mg12Nd particles in the WE43 alloy also serve as cathodes with respect to the matrix [7].
The formation of Mg24Y5 precipitates in HCP-Mg matrix during heat treatment caused the lowering of the corrosion resistance of the alloys [32].
Mg2Si seems to have no effect on the corrosion of Mg alloys.
3.1.3 Grain size
The rapid solidification process can refine the microstructure which is beneficial to the corrosion properties. It can change the mechanism of corrosion; turning pitting corrosion of Mg-Al magnesium alloys into overall corrosion [33]. The surface or skin layer of die-cast Mg-Al magnesium alloys with very fine grains, high β volume fraction and continuous distribution of β phase along grain boundaries has a higher corrosion resistance than its core [27]. It is the same that die castings of magnesium alloy AZ91D have better corrosion resistance than ingots [27].
3.2 Effects of post processing
3.2.1 Heat treatment
Heat treatment can change the microstructure of magnesium alloys. Aging makes Al atoms diffuse towards grain boundaries and form precipitation of the β phase, thus, reducing the aluminum concentration in the α-Mg matrix. For example, the aluminum content in the α-Mg matrix of Mg-9Al alloy decreases from 9% to 3% [2]. LEFEBVRE et al [34] studied AZ91 and Mg-7.5Al and found that weight corrosion rate for T6 treating was only (1.1±0.4)mm/a, and less than 3.2 mm/a and 4.7 mm/a, separately, for T4 treating immersion in 3.5%NaCl aqueous solution. ELIEZER et al [6] and LUNDER et al [29] investigated the corrosion of AZ91by different heat treatments (F, T4 and T6 treating) and found that the corrosion rate decreased in the sequence T4, F and T6 treating. Studies [6, 34, 35] showed that corrosion resistance decreased in the sequence: Mg17Al12>after T6 treating>after T4 treating. Rapidly solidified (RS) Mg97.16Zn0.92Y1.92 alloy exhibited high corrosion resistance, but their corrosion rates increased with increasing the heat treatment temperature [32].
Ageing reduces corrosion resistance of Mg-RE alloys. For instance, the corrosion resistance of Mg-Zn-Y RS alloy decreased due to Mg24Y5 precipitation caused by heat treatment [32].
ageing treatment of AZ80 has little influence on the fatigue life in air and in corrosive media at higher stress level, but improves remarkably the fatigue life in corrosive media at lower stress level [23].
3.2.2 Effects of welding
HAFERKAMP et al [36] made some investigations on the corrosion behavior of laser welded AZ91D in synthetic seawater. Fig.4 shows the corrosion morphology of the weld zone of AZ91D weld with gas tungsten arc (GTA) welding after 48 h salt fog test. The corrosion morphology of the weld zone of AZ31 with laser beam welding after 24 h salt frog test is shown in Fig.5. It is visible that the corrosion does not attack the weld zone.
The polarization curves of AZ91D weld and its base materials (shown in fig.6) indicate that the corrosion rate of weld zone is half of that of base material due to its
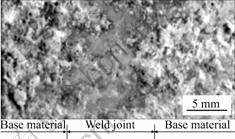
Fig.4 Corrosion morphology of weld zone of AZ91D with GTA welding
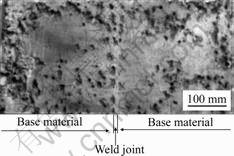
Fig.5 Corrosion morphology of weld zone of AZ31 with laser beam welding
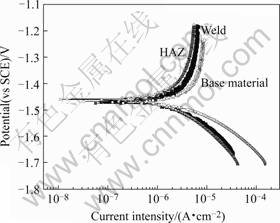
Fig.6 Polarization curves of AZ91D weld and its base materials in 3.5% NaCl solutions
finer grain size.
The high welding speed and fast cooling rate of the welds could improve the corrosion resistance of the weld zone connected with the same material because of its fine grain sizes and of solid solutions with higher aluminum content [37].
The resistance of non-welded alloys increases with the increase of Al content. On welding of the alloys, the corrosion resistance is determined by the Al/Mg proportion at the surface of the non-affected material and the laser-welding seam. The pits occur mainly in the heat-affected zone of the welding seam. The laser beam welded material AZ61HP was found to have excellent resistance [38].
SKAR et al [39] investigated the corrosion properties of weld AM50A and AZ90D. The tests showed that the weld materials had poor corrosion resistance, and the corrosion rate of AM50A was higher than that of AZ91D. The AZ91D/AM50A welds showed corrosion rates comparable to AM50A alone. Embedded iron contaminants from wearing of the tool may be responsible for the poor corrosion properties of the welds. Partially melting of the β phase that easily dissolves iron may cause this wearing.
Therefore, the corrosion tendency of rapidly solidified welds, as in the case of high power laser welding, is relatively low. As for dissimilar welding of magnesium and aluminium alloys, Mg and weld seams neighboring the Mg side will corrode because of their lower potential. The corrosion behavior of Mg weld zone and the effects of the welding process parameters on corrosion, stress corrosion cracking and corrosion fatigue, however, are not well known and further research remains to be done.
3.3 Environmental influence
3.3.1 environmental temperature and relative humidity
Corrosion of magnesium alloys increases with the increase of relative humidity (RH). At 9.5% RH, neither pure magnesium nor any of its alloys exhibit evidence of surface corrosion after 18 months. At 30% humidity, only minor corrosion may occur. At 80% humidity, the surface may exhibit considerable corrosion. In marine atmospheres heavily loaded with salt spray, magnesium alloys require protection for prolonged survival [40].
SCC is affected by elevated environmental temperature and relative humidity. The susceptibility of SCC increases with the raised temperature. Creep deformation can improve SCC resistance. high humidity accelerates SCC during atmospheric exposure [8].
The fatigue life is reduced, and the FCP rate is enhanced by the increasing environmental temperature and relative humidity (RH). The fatigue limit of as-extruded AZ61 lay between 145-150 MPa in 55% RH at 20 ℃ and 50 ℃. Corrosion fatigue (CF) occurred in 80% RH in air[41].
The RH affected not only the fatigue life but also the FCP rate in air. The FCP rates of Mg alloys increased remarkably with the increase of RH in air [41]. Atmospheric moisture accelerates fatigue crack growth and decreases the threshold stress intensities to 55%-75% of the respective values in vacuum. In ambient air, fatigue crack growth rates were up to two decades higher than those in vacuum [42].
many authors [9] have illustrated the influence of several environmental media on fatigue crack propagation rates of magnesium alloys. Even the increase of humidity, in air or argon leads to an increase of the fatigue crack propagation rate in AZ91, AZ61and AM60, showing a very high sensitivity of magnesium alloys to humidity.
3.3.2 Electrolyte pH value
The pH value of the medium has an important impact on the corrosion morphology and the number of pits. As a result, the corrosion of magnesium alloys in neutral or alkaline salt solutions typically takes the form of pitting [2, 40]. It can resist corrosion in alkaline solution, in particular, when the pH value is higher than 11.5, corrosion does hardly occur [2, 35]. The corrosion rate of the β phase is very low when pH value is 4-14. Analysis by Auger electron spectroscopy (AES) showed that the thickness of the oxide film of the β phase formed in solution with pH=12 was several times that formed in air, increasing with decreasing pH value [30]. The corrosion rate of AZ91 ingot and die-cast is high in acidic solutions (pH=1-2) as compared to that in neutral and highly alkaline solutions (pH=4.5-12) [28]. However, it is different from that of as-extruded magnesium alloy AM60 in 3.5% NaCl solutions at all pH levels. The pitting corrosion morphologies for as-extruded magnesium alloy AM60 in 3.5% NaCl solutions at pH=3, pH=7 and pH=12 are shown in Fig.7 [5].
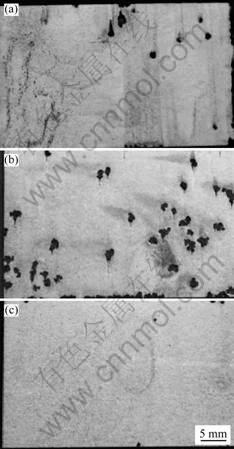
Fig.7 Corrosion morphologies of AM60 as extruded in 3.5%NaCl aqueous solution at pH=3(a), pH=7(b) and pH=12(c), respectively[5]
It reveals that more pitting corrosion occurs at pH=7, while no pit, except on the edge, occurs at pH=12. It is found by EDS that pits initiate at AlMn particles, and high Al concentration areas dissolve at pH=12 and its morphology is in the form of a web [5]. The number of average corrosion pits for AM60 extrusion is highest at pH=7. Thus, fatigue life of extruded AM60 is the shortest in neutral solution and the longest in base solution with pH=12[22].
Corrosion resistance of magnesium alloys with high RE concentration is the best in 3.5% NaCl with pH=11, and the poorest in ASTM D-1384 solution(composition:148 mg/L Na2SO4, 165 mg/L NaCl, 138 mg/L NaHCO3, pH0=8.8) at pH=8.8 [43]. Corrosion fatigue life of magnesium alloys depends highly on the pH values of the solution [44]. The SCC rates of magnesium alloy (6.5%Al,1%Zn,0.2%Mn) increase significantly in NaCl-KCrO4 solution at pH<5; the SCC rate remains stable at pH=5-12;the SCC rate reduces at pH>12[40].
3.3.3 Chloride ion concentration
Extruded magnesium alloys with 3% to 8% Al and 0.5%-0.8% Zn are susceptible to filiform corrosion and pitting corrosion in aqueous chloride solutions depending on chloride concentration [45]. AMBAT et al [28] found that the corrosion rate increased with the increase of chloride ion concentration at all pH levels. The open circuit corrosion potential shifted to more negative values with the increase of concentration of chloride ions. NAKATSUGAWA et al [46] studied the influence of the chloride ion concentration on the corrosion behavior of thixomoulded AZ91D alloy under anodic polarization and natural immersion, and a small passivation following its breakdown at critical potential was observed on anodic polarization curves. The critical potential showed a linear relationship with the square root of the scanning rate and the logarithm of the sodium chloride concentration. The critical concentration for depassivation was estimated to around 10 mol/m3. if it was lower than this value, the corrosion rate showed a constant value around 0.03 mm/a. Cathodic ions (Cl-, Br-, I- and ![]() ) facilitate fatigue crack propagation rates (FCPR) of magnesium alloys [1].
) facilitate fatigue crack propagation rates (FCPR) of magnesium alloys [1].
4 Outlook
From the point of view of corrosion protection, alloying elements and impurities of Mg alloys have to be controlled according to the endurance limit. Mg producers usually add more manganese to reduce the iron content in the melting. Much more manganese, however, is also detrimental to the corrosion of Mg alloys. Mn, reacting with Al, forms AlMn (Fe) phase, which has a highest noble potential except Al3Fe (Mn) in the secondary phases; at the same time it decreases the formation of Mg17Al12 phase.
The corrosion mechanisms of magnesium alloys are still not well understood. Therefore, it is definitely necessary to continue this investigation.
Research on the corrosion behavior of Mg welds and on the effect of welding process parameters on corrosion, particularly SCC and CF, remains to be done.
There is a challenge for corrosion scientists to create a perfect coating on Mg alloys. It is essential for the coating to have sufficient adhesion on magnesium, high hardness and mechanical strength, good toughness, environmental friendliness, excellent corrosion resistance, and even better fatigue and wear resistance. But, so far there are no coatings that can satisfy all these demands. Metallic coatings such as Ni, Cr and Al etc. have higher hardness and mechanical properties. The Cr process produces poisonous solutions to environment. A Ni film will facilitate the galvanic corrosion, when it is damaged during assembly. It also enhances the difficulty of recyclable use. Perhaps, Al coatings have a potential for wider application. Non-metallic coatings comprise phosphate conversation films, polymers etc. These films are very thin and soft, and have less mechanical strength and hardness to fit an assembly, and are easy to crack. It is possible for these coatings to become a primer, and then a suitable surface paint needs to be applied.
References[1] MAKER G L, KRUGER J. Corrosion of magnesium [J]. International Material Review, 1993, 38: 138-153.
[2] SONG G, ATRENS A. Corrosion mechanisms of magnesium alloys [J]. Advance Engineering Materials, 1999,1: 11-33.
[3] SONG G, ATRENS A. Understanding magnesium corrosion [J]. Advance Engineering Materials, 2003, 5(12): 837-858.
[4] SONG G. Recent progress in corrosion and protection of magnesium alloys [J]. Advance Engineering Materials, 2005, 7(7): 563-586.
[5] ZENG Rong-chang, ZHOU Wang-qiu, HAN En-hou, KE Wei. effect of pH value on corrosion of as-extruded AM60 magnesium alloy [J]. Acta Metallurgica Sinica, 2005, 44(3): 307-311.(in Chinese)
[6] ELIEZER D, UZAN P, AGHION E. Effect of second phases on the corrosion behavior of magnesium alloys [J]. Material Science Forum, 2003, 419-422: 857-866.
[7] GUO L F, YUE T M, MAN H C. Excimer laser surface treatment of magnesium alloy WE43 for corrosion resistance improvement [J]. Journal of materials science, 2005, 40: 3531-3533.
[8] GHALI E. corrosion and protection of magnesium alloys [J]. Materials Science Forum, 2000, 350-351: 261-272.
[9] GHALI E, DIETZEL W, KAINER K U. General and localized corrosion of magnesium alloys: a critical review [J]. Journal of materials engineering and performance (JMEPEG), 2004, 13(1): 7-23.
[10] Zeng Rong-chang, Ke Wei, Xu Yong-bo, Han En-hou, Zhu Zi-yong. Recent development and application of magnesium alloys [J]. Acta Metallurgica Sinica, 2001, 37(7): 673. (in Chinese)
[11] TONG Zhen-song, ZHANG Wei, LI Jiu-qing, CHENG Fei. Initial laws of atmospheric galvanic corrosion for magnesium alloys [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(4): 554-561. (in Chinese)
[12] SKAR J I. Corrosion and corrosion prevention of magnesium alloys [J]. Materials and corrosion, 1999, 50: 2-6
[13] VALENTE T. Grain boundary effects on the behavior of WE43 magnesium castings in simulated marine environment [J]. Journal of Materials Science Letters, 2001, 20: 67-69.
[14] LUNDER O, LEIN J E, HESJEVIK S M, AUNE T KR, NISANCIOGLU. Corrosion morphologies on magnesium alloy AZ91 [J]. Werkstoffe und Korrosion, 1994, 45: 331-340.
[15] DEXTER S C. Metals Handbook(Vol.13)[M]. 9th ed. OH: ASM International, 1987. 106.
[16] WILLIAM K, MILLER. Stress-corrosion Cracking [M]. Ohio: ASM, 1993. 251.
[17] WINZER N, ATRENS A, SONG G, GHALI E. DIETZEL W, KAINER K U, HORT N, BLAWERT C. A critical review of the stress corrosion cracking (SCC) of magnesium alloys [J]. Advanced Engineering Materials, 2005, 7(8): 659-693.
[18] SONG R G, BLAWERT C, DIETZEL W, ATRENS A. A study on stress corrosion cracking and hydrogen embrittlement of AZ31 magnesium alloy [J]. Mater Sci Eng A, 2005, A399: 308-317.
[19] POTZIES C, KAINER U K. Fatigue of magnesium alloys [J]. Advanced Engineering Materials, 2004, 6(5): 281-289.
[20] YUE T M, HA H U, MUSSON N J. Grain size effects on the mechanical properties of some squeeze cast light alloys [J]. Journal of Material Science, 1995, 30: 2277-2283.
[21] STEPHENS R I, SCHRADER C D, LEASE K B. Corrosion fatigue of AZ91E-T6 cast magnesium alloy in a 3.5 percent NaCl aqueous environment [J]. Journal of Engineering Material and Technology, 1995, 117(7): 293-298.
[22] Zeng Rong-chang, Han En-hou, Ke wei, Liu Lu, Xu Yong-bo. Corrosion fatigue of as-extruded AM60 magnesium alloy[J]. Chinese Journal of Materials Research, 2005, 19(1): 1-7. (in Chinese)
[23] Zeng Rong-chang, Han En-hou, Ke wei, Xu Yong-bo, Liu Lu. mechanism of corrosion fatigue for as-extruded magnesium alloy AZ80[J]. Chinese Journal of Materials Research, 2004, 18(6): 561-567. (in Chinese)
[24] DANIELSON M J, JONES R H. The interaction between microstructure and corrosion initiation in certain die cast and Thixomolded? magnesium alloys [A]. Magnesium Technology 2001[C]. New Orleans: TMS, 2001. 263-268.
[25] SONG G, STJOHN D. The effect of zirconium grain refinement on the corrosion behavior magnesium-rare earth alloy MEZ [J]. Journal of the Light Metals, 2000, 2: 1-16.
[26] Scharf C , Ditze A, Shkurankov A, Morales E, Blawert C, Dietzel W , Kainer KU. Corrosion of AZ91 secondary magnesium alloy [A]. Advanced Engineering Materials, 2005, 7(12): 1134-1142
[27] SONG G, ATRENS A, DARGUSCH M. Influence of microstructure on the corrosion of die cast AZ91D [J]. Corrosion Science, 1999, 41: 249-273
[28] AMBAT R, AUNG N N, ZHOU W. Evaluation of microstructural effects on corrosion behavior of AZ91D magnesium alloy [J]. Corrosion Science, 2000, 42: 1433-1455.
[29] LUNDER O, LEIN J E, HESJEVIK S M, et al. The role of Mg17Al12 phase in the corrosion of Mg alloy AZ91 [J]. Corrosion, 1989, 45(9): 741-748.
[30] KO Y J, YIM C D, LIM J D, SHIN K S. Effect of Mg17Al12 precipitate on Corrosion behavior of AZ91D magnesium alloy [J]. Mater Science Forum, 2003, 419-422: 851-856.
[31] MATHIEU S, RAPIN C, STEINMETZ J, STEINMETZ P. A corrosion study of the main constituent phases of AZ91 magnesium alloys [J]. Corrosion Science, 2003, 45: 2741-2755.
[32] YAMASAKI M, NYU K, KAWAMURA Y. corrosion behavior of rapidly solidified Mg-Zn-Y Alloy ribbons [J]. Materials Science Forum, 2003, 419-422: 937-942.
[33] DALOZ D, STEINMETZ P, AND MICHOT G. Corrosion behavior of rapidly solidified magnesium-aluminum-zinc alloys [J]. Corrosion, 1997, 53(12): 944-954.
[34] LEFEBVRE F, NUSSBAUM G. Extraction, Refining and Fabrication of Light Metals [M]. Ontario: Pergamon Press, 1991.19-31.
[35] BELDJOUDI T, FIAUD C, ROBBIOLA L. Influence of homogenization and artificial aging heat treatments on corrosion behavior of Mg-Al alloys [J]. Corrosion. 1993, 49(9): 733-745.
[36] HAFERKAMP H, NIEMEYER M, SCHMID C, KAESE V, CORDINI P. Laser welding of magnesium alloys-cooling conditions and resulting metallurgical properties [A]. magnesium 2000 [C]. Beer-Sheva: Magnesium Research Institute LTD, 2000. 449-455.
[37] CAO X, JAHAZI M, IMMARIGEON J P, WALLACE W. A review of laser welding techniques for magnesium alloys [J]. Journal of Materials Processing Technology, 2006, 171: 188-204.
[38] GHALI K LUEBBERT, J KOPP E. Wendler-Kalsch. Corrosion behavior of laser beam welded aluminum and magnesium alloys in the automotive industry [J]. Mater Corrosion, 1999, 50: 65-72.
[39] SKAR J I, GJESTLAND H, OOSTERKAMP L D, ALBRIGHT D L. Friction stir welding of magnesium die castings [A]. Magnesium Technology 2004[C]. North Carolina: TMS, 2004. 25-30.
[40] FROATS A, AUNE T K, HAWKE D, UNSWORTH W, HILLIS J. Metals Handbook 13th ed. [M]. Ohio: ASM, 1987. 740.
[41] ZENG Rong-chang, HAN En-hou, KE Wei. Fatigue and corrosion fatigue of magnesium alloys [J]. Material Science Forum, 2005, 488-489: 721-724.
[42] PAPAKYRIACOU M, MAYER H, FUCHS U, STANZL-TSCHEGG S E WEI R P. Influence of atmospheric moisture on slow fatigue crack growth at ultrasonic frequency in aluminum and magnesium alloys [J]. Fatigue & Fracture of Engineering Material & Structure, 2002, 25: 795-804.
[43] MORALES E D, GAHLI E, HORT N, DIETZEL W, KAINER K U. Corrosion behavior of magnesium alloys with RE additions in sodium chloride solutions [J]. Mater Science Forum, 2003, 419-422: 867-872.
[44] ELIEZER A, GUTMAN E M, ABRAMOV E, UNIGOVSKI Y. Corrosion fatigue of die-cast and extruded magnesium alloys [J]. Journal of the Light Metals, 2001, 1(3): 179-186.
[45] L?BBERT K, KOPP J, WENDLER-KALSCH E. Corrosion behavior of laser beam welded aluminum and magnesium alloys in automotive industry [J]. Materials and Corrosion, 1999,50: 65-72.
[46] NAKATSUGAWA I, TAKAYASU H, ARAKI K, TSUKEDA T. Electrochemical corrosion studies of thixomolded AZ91D alloy in sodium chloride solution [J]. Mater Sci Forum, 2003, 845-850: 419-422.
Foundation item: Project (CSTL, 2004BA4002 and 8655) supported by the Key Natural Science Foundation of Chongqing Municipal Science and Technology Committee, China; Project (KJ050604) supported by the Science and Technology Program of Chongqing Municipal Education committee, China
Corresponding author: ZENG Rong-chang, Tel: +86-23-68665616; E-mail: rczeng2001@yahoo.com.cn

