
Nickel co-deposition with SiC particles at initial stage
TAN Cheng-yu(谭澄宇), LIU Yu(刘 宇), ZHAO Xu-shan(赵旭山), ZHENG Zi-qiao(郑子樵)
College of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 3 September 2007; accepted 14 December 2008
Abstract: The cyclic voltammetry and chronoamperometry were used to study the influence of SiC particles on the nucleation and growth of nickel deposition on copper matrix from acid sulphate solution. The surface morphology of Ni-SiC co-deposition at initial stage was observed with scanning electron microscope(SEM). The results show that the nickel co-deposition with SiC particles may begin at -700 mV (vs SCE) under the experimental conditions, and the nucleation/growth mechanism of Ni-SiC co-deposition tends Scharifker—Hill with three-dimension model. In the case of low over-potential of -710--740 mV, the nucleation process of Ni-SiC co-deposition may follow the progressive nucleation mechanism of Scharifker—Hill. During higher over-potential of -770--800 mV, it trends to follow a 3D instantaneous nucleation/growth mechanism. With the increase of over-potential, the relaxation time tm, corresponding to the peak current Im of Ni-SiC co-deposition decreases regularly and is shortened apparently, compared with that of pure Ni deposition. The observation with SEM confirms that SiC particles can be considered as favorable sites for nickel nucleating. When certain amount of SiC particles are adsorbed (or cover) on cathode surface, especially under the condition of high over- potential, they may represent an external inhibition of the nickel electron deposition.
Key words: Ni-SiC film; electron crystallization nucleation; co-deposition mechanism; I—t curve
1 Introduction
The composite coatings usually made up of solid grain and matrix metal have good properties for various applications and have attracted people’s attention. At present, many literatures about preparation method, process and properties of various composite coatings have been published[1-5] in recent years. Several researchers[6-9] have also studied the mechanics on metal co-deposition with solid particles. But few papers[10-11] on electron-crystallization behaviors of composite co-deposition were reported by using electrochemical measurement technique.
The electron crystallization process mainly relates to nucleation and growth of electron deposition. At present, there are many electron crystallization mathematic models on metal electron deposition process, including 2D disk mode, 3D hemisphaerium shape and regular cone mode, etc. Three-dimensional semi-sphere mode and three-dimensional code mode are widespread recognized[12-16]. GOMEZ et al[17] studied the initial stage of electro-crystallization behaviors in NiCl solution system with constant step voltage technique and cyclic voltammetry. They considered that Ni electro- crystallization nucleation and growth under low over-potentials followed continuous nucleation mode. Under high over-potentials, they meet instantaneous nucleation of three-dimensional mode. The study on electron-crystallization behaviors of composite deposition may be favorable to understand the effect of solid particles on electron-crystallization process and analyses of the microstructure and properties of the composite coatings[18-19].
In this study, an attempt was made to clarify the effect of silicon carbide particles during co-deposition. The electron-crystallization behaviors of Ni-SiC co-deposition process and the influence of SiC particles on the nucleation process were investigated by using cyclic voltammetry and chronoamperometry technique with hope to offer favorable help and reference for composite coating application and research in the future.
2 Experimental
Ni-SiC composite films were electrodeposited onto the copper substrates (d 2.70 mm) from Watts-type electrolyte consisting of nickel sulfate NiSO4·6H2O, nickel chloride NiCl·6H2O, boric acid H2BO3 and silicon carbide powders (25 g/L SiC). The experimental electrolyte was prepared with AR grade reagents and distilled water, and pH value of the solution was adjusted to 3.8-3.9 using H2SO4 and NaOH reagent. The silicon carbide particles were added to the bath and held in suspension by applying the bath agitation using a magnetic stirrer. The experimental temperature was (50 ±1) ℃ controlled by thermostat water tank.
A standard three-electrode configuration consisting of a sample as the working electrode(WE), a conventional saturated calomel reference electrode(SCE) and a large platinum foil with an area of 4 cm2 as the counter electrode was used to measure the cyclic voltammetry and chronoamperometry curves of electro- deposition on copper matrix. All electrochemical tests were performed with a commercial model CHI 660C electrochemical analyzer/workstation. Before testing, the exposed surface of the working electrode was polished with silicon carbide papers, rinsed with the distilled water, washed in acetone, rinsed with the distilled water again and then dried in air. The current—time curves were measured in the range from -230 to -890 mV at a step of 30 mV and the potential step began from zero potential (referred to SCE). Before measurement, the bath solution would hold still stabilization for 30 min or so. (I/Imax)2—t/tmax diagrams of Ni or Ni-SiC electron deposition were plotted from corresponding experimental I—t curves.
The surface morphologies of various deposition films were also observed with Sirion200 type emission scanning electron microscope(SEM). The compositions on the film surface were analyzed with Genesis60S type energy detective spectrometer(EDS). The influence of SiC particles on Ni electro-deposition process was analyzed and discussed.
3 Results and discussion
3.1 Chronoamperometry for nucleation modeling
In general, the electro-deposition nucleation at initial stage undergoes a series of process such as absorbing or inducing atoms on cathode surface, gathering to atom clusters and formatting new nucleation with critical size. At present, there are two classic mechanism for describing the electro-crystallization process, i.e. BFT and SHS nucleating models[15-17] with three-dimensional growth fashion. Supposing crystal nucleus as original cone shape and their growth in 3D fashion, electro-deposition rate is controlled by absorbed atoms moving into crystal lattice points at initial stage of electro-crystallization. According to BFTs’ models, the initial transient current is given as follows:
Instantaneous nucleation:

Progressive nucleation:
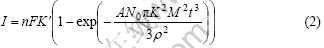
where K and K′ represent the growth rates along the parallel matrix surface and in the direction of vertical matrix surface respectively, F is the Faraday constant, n is the number of electrons involved in the chemical process, M is the molecular mole mass, A is the nucleation rate constant, ρ is the density, t is the time, and N and N0 are the crystal nucleus density and the maximum density of crystal nucleus number or active site number on surface, respectively.
Scharifker—Hill model based on the assumption of the growth of crystalline nucleus (random distribution on matrix electrode) as semi-sphere shape may be controlled by diffusion process. No new nucleus are formed in the extended growth area. Overlap extended area may also be considered. So, the equations describing transience current are expressed as follows:
Instantaneous nucleation:
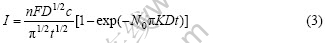
Progressive nucleation:

where D is the diffusion coefficient and c is called the molar concentration. Two equations mentioned above give the transient current of instantaneous or progressive nucleation, respectively. The important information on metal electron crystallization nucleation and growth can be obtained by analyzing the I—t curves of electron deposition. When the step-potential changes from OCP (open circuit potential) to metal deposition potential, the reaction process may gradually drive to steady-state reaction (controlled by mass transport process). The steady-state current is given by Cottrell equation as follows:

where nF is the molar charge for nickel ion and c0 is the solution concentration. The non-dimension expressions for instantaneous and progressive nucleation can be derived from Eqns.(1)-(4). We can analyze I—tn curves and non-dimension (I/Imax)2—t/tmax curves according to BFT model and Scharifker—Hill model synthetically and may determine the nucleation fashion for electro-crystallization process.
3.2 Cyclic voltammetry
Fig.1 shows the cyclic voltammetry(CV) curves of pure Ni and Ni-SiC electron deposition. It can be seen from Fig.1 that there is a visible reduction current peak on CV curves at applied potential -350 mV (vs SCE) or so. The current peak may be a pre-peak that depends on pH change of bath solution according to the literature reported by CAO[20]. The experiments in this study confirm that no electric current of the nucleation and growth occur during potential from OCP (open circuit potential) to -680 mV (vs SCE). Appling -700 mV (vs SCE) just shows a visible electric current peak (as arrowhead notation) of nickel deposition and Ni-SiC deposition current may be a little larger than that of pure Ni deposition. In the range of -800--1 000 mV, the electric current gradually increases with increasing potential. The current of pure Ni deposition exceeds that of Ni-SiC deposition, which may reflect the influence of SiC particles on the nucleation and growth process of nickel electron deposition.
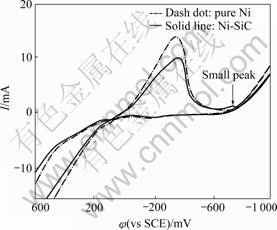
Fig.1 Cycle voltammograms for nickel co-deposition with SiC particles (scan rate: 100 mV/s)
3.3 Potentiostatic current transient
Fig.2 shows the current—time curves of nickel or Ni-SiC deposition on pure copper electrode under various step potentials. It can be found from Fig.2 that I—t curves of Ni-SiC co-deposition are similar to pure nickel deposition curves. In Figs.2(a) and (b), the electric electric current decreases rapidly because of charge on electric double layer of the electrode surface. In Figs.2(c) and (d), the current firstly rises, and then decreases (the current controlled by diffusion on electrode surface) due to the beginning of nucleation and new phase growth at -680--710 mV. With negative step potential, the relaxation time tm, corresponding to peak current is shortened gradually, which reflects the rapid nucleation and growth for electron crystallization process under high over-potential. In the range of -710 mV--800 mV, corresponding to the small peak current in Fig.1, the current of Ni-SiC deposition is a little larger than that of pure Ni deposition.

Fig.2 Potentiostatic current transients for nucleation and growth on copper electrode under various applied potentials: (a) -230- -320 mV; (b) -470- -590 mV; (c) -710- -740 mV; (d) -770- -830 mV
Table 1 lists the maximum current and corresponding time respectively for pure nickel and Ni-SiC deposition under various step potentials according to above experimental curves in Figs.2(c) and 2(d).
Table 1 Imax and tmax of transient curves for nucleation and growth
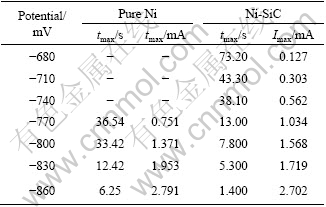
The beginning potential of Ni-SiC deposition may be ahead of pure nickel beginning crystalline potential. With over-potentials, the relaxation time tmax of Ni-SiC co-deposition will reduce gradually (in exponential decay relation). In the same applied potential, the time tmax for Ni-SiC deposition may be shorter than that of pure Ni deposition, which may imply that SiC particles on cathode surface can accelerate the nucleation process. It can also be found from Table 1 that at -770 and -800 mV, the maximum current of Ni-SiC co-deposition is a little larger than that of pure nickel deposition.
Fig.3 shows some non-dimensional (I/Imax)2—t/tmax curves of pure Ni and Ni-SiC electro deposition on pure copper electrode under various step potentials. In Fig.3, the test data (on the uplifted side of the current—time curve) may locate in the range between progressive and instantaneous nucleation of SHE model, indicating that the nucleation mechanism would prefer Scharifker— Hill 3D growth model than Bewick-Fleshmann-Thrisk growth model.
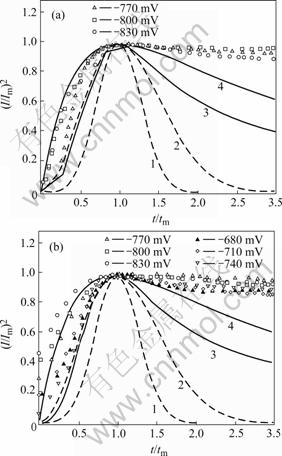
Fig.3 Non-dimensional (I/Im)2 vs t/ tm plots corresponding to Fig.2: (a) Pure Ni deposition; (b) Ni-SiC deposition (Dash—BFT model, Solid—SHE model; 1,3—Progressive; 2,4—Instantaneous)
In Fig.3(a), under -770 mV, the nucleation mechanism of pure nickel deposition can approach to the progressive nucleation fashion. With over-potential increment, the nucleation fashion of pure nickel deposition may meet the instantaneous nucleation of Scharifker—Hill model and the nucleation or growth current may be controlled by diffusion behavior on electrode surface. The experimental results accord with the work of GOMEZ et al[17]. The non-dimensional curves of nickel co-deposition with SiC particles are similar to those of pure nickel deposition. In Fig.3(b), under -680--740 mV, the nucleation fashion of Ni-SiC co-deposition may be near to the progressive nucleation model, and with over potential, Ni-SiC co-deposition may undergo the transition change from the progressive to the instantaneous nucleation model.
In the range of -770--830 mV, the test data on the uplifted side is very near to instantaneous nucleation theoretical curves, which indicates that under high over potential, the nucleation of Ni-SiC co-deposition follows instantaneous nucleation of Scharifker—Hill model. At -830 and -860 mV, the test points on the uplifted side may locate outside the nucleation model curves and deviate from the instantaneous model to some extent.
3.4 SEM observation for co-deposition at initial stage
Fig.4 shows the SEM images of Ni co-deposition with SiC particles for several seconds on copper matrix under the conditions of the current density 1 A/dm2, high over-potential (actual tank voltage about 1 100 mV) and the bath solution containing 25 g/L silicon carbide powders.
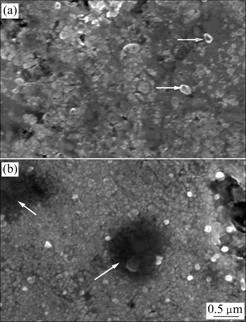
Fig.4 SEM images of Ni-SiC co-deposition on pure copper matrix: (a) 5 s; (b) 10 s
After the sample plated for 5 s (Fig.4(a)), there are many very small crystal nucleus and a few small SiC particles (marked with two arrows in Fig.4(a)) on copper matrix surface. But some regions are naked matrix and there are still no crystal nucleus. After plated for 10 s, a great deal of nucleus that overlap each other are formatted on matrix surface and the nucleation-free zone decreases apparently. In Fig.4(b), there are two dark regions (marked with two arrows) apparently which may be caused by the coarse particles separating off. It is found from the SEM images that SiC particles may refine the microstructure morphology of the composite coating.
Nickel co-deposition nucleations from the bath solution containing SiC particles are complicated because of the influence of SiC particles on the deposition process. In the case of lower over-potential, it is considered from the nucleation interfacial energy that SiC particles on cathode surface may be favorable sites for the deposition nucleation to reduce the over-potential of the deposition nucleation and promote electron crystallization nucleation[9-10]. So, the beginning potential of Ni-SiC deposition may be ahead of pure nickel beginning crystalline potential. At initial stage, the corresponding peak current Imax of Ni-SiC deposition may be a little larger than that of pure nickel deposition. With the increase of over potential, the number of active sites for the nucleation on surface may enhance the deposition nucleation. The influence of SiC particles on the deposition process may shorten the nucleation time tm gradually and the nucleation time of Ni-SiC co-deposition is shorter than that of pure Ni deposition. It is found through fitting test curves that E—tm curves for Ni and Ni-SiC deposition meet exponential decay law and the correlation coefficients are about 0.9.
Fig.5 shows the sketch for describing the influence of SiC particles on the nucleation process of Ni deposition. Several sites for the nucleation crystallization are indicated as 1-5 in Fig.5. It is considered from minimal interfacial energy that SiC particle surface may be favorable site for the nucleation. The analysis with EDS confirms that there is nickel element on SiC particles surface, showing that SiC particles may engage in the nucleating process.
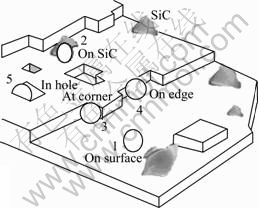
Fig.5 Sketch of metal atoms depositing at various activity positions: (○—Nickel ion; 1, 2, 3, 4, 5—Various positions for nickel deposition)
Under higher over-potential, the nickel nucleation rate is high and there are more active sites for the nucleation on the electrode surface. If a large number of SiC particles are adsorbed or accumulated on matrix surface, because of their poor conductivity, they may mask matrix surface, decrease effective cathode surface and obstruct mass transmission for the nickel deposition (SiC on electrode surface may barricade the electric discharge of partial Ni2+ and Ni2+ transmission for Ni2+ reducing reaction), which leads to a little descending peak current. So, under the condition of high over-potential such as -830 mV, SiC particles congregated on the cathode surface may be a hindrance to the growth process. SiC particles on cathode surface will have a very complicated influence on the co-deposition process and the microstructure morphology of composite coating.
4 Conclusions
1) The nickel co-deposition with SiC particles begins at -700 mV (vs SCE). The nucleation time of Ni-SiC deposition may gradually be shortened with over potential increasing and be more shorter than that of pure nickel deposition, which reflects that SiC particles on the electrode surface may offer many favorable sites for nickel crystallization nucleation. But SiC particles may also mask matrix surface, and obstruct mass transmission for the nickel deposition, which leads to a little descending peak current.
2) The nucleation of Ni-SiC co-deposition at initial stage follows 3D Scharifker—Hill mode. In the case of low over-potential (-680--740 mV), the nucleation fashion meets progressive nucleation. With increasing the applied potential, Ni-SiC electrodeposition may undergo the transition change from progressive to instantaneous nucleation. Under the condition of higher over-potential (-770--830 mV), Ni-SiC co-deposition may meet instantaneous nucleation mechanism.
3) SiC particles can not alternate the nucleation mechanism of nickel deposition on matrix surface, but have an influence on deposition process and can refine the microstructure morphology of the composite coating.
References
[1] BENEA L, BONORA P L, BORELLO A, MARTELLI S, WENGER F, PONTHIAUX P, GALLAND J. Preparation and investigation of nano-structured SiC-nickel layers by electrodeposition [J]. Solid State Ionics, 2002(151): 89-95.
[2] LI J, SUN Y, SUN X, QIAO J. Mechanical and corrosion-resistance performance of electrodeposited titania-nickel nano-composite coating [J]. Surface & Coatings Technology, 2005(192): 331-335.
[3] PENG X, ZHANG Y, ZHAO J, WANG F. Electrochemical corrosion performance in 3.5% NaCl of the electrodeposited nanocrystalline Ni films with and without dispersions of Cr nanoparticles [J]. Electrochimica Acta, 2006(51): 4922-4927.
[4] WANG LIQIN, WU HUA, TIE JUN, YAN Chuan-wei. Preparation technology and properties of electrodeposited Ni/nano SiC composite coatings [J]. Corrosion Science and Protection Technology, 2005, 17(14): 230-233.
[5] WANG Hong-zhi, YAO Su-wei. Electrochemical preparation and characterization of Ni/SiC gradient deposit [J]. Journal of Materials Processing Technology, 2004, 145(3): 299-302.
[6] HU F, CHAN K C. Equivalent circuit modelling of Ni-SiC electrodeposition under ramp-up and ramp-down waveforms [J]. Materials Chemistry and Physics, 2006, 99(2/3): 424-430.
[7] LEE H K, LEE H Y, JEON J M. Codeposition of micro- and nano-sized SiC particles in the nickel matrix composite coatings obtained by electroplating [J]. Surface and Coatings Technology, 2007, 201(8): 4711-4717.
[8] LIN C S, HUANG K C. Co-deposition and microstructure of nickel-SiC composite coating electrodeposited from sulphamate bath [J]. Journal of Applied Electrochemistry, 2004(34): 1013-1019.
[9] HU F, CHAN K C. Deposition behavior and morphology of Ni-SiC electro-composites under triangular waveform [J]. Applied Surface Science, 2005, (243): 251-258.
[10] NOWAK P, SOCHA R P, KAISHEVA M, FRANSAER J, CELIS J P, STOINOV Z. Electrochemical investigation of the co-deposition of SiC and SiO2 particles with nickel [J]. Journal of Applied Electrochemistry, 2000(30): 429-437.
[11] SANDRA W W. Electrochemical study of SiC particle occlusion during nickel electrodeposition [J]. J Electrochem Soc, 1993, 140(8): 2235-2238.
[12] ZHA Quan-xing. Guide to dynamics of electrode process [M]. 3rd Edition. Beijing: Scientific & Technical Press, 2004: 308-310.
[13] BEWICK A, FLEISCHMANN M, THIRSK H R. Kinetics of the electrocrystallization of thin films of calomel [J]. Transactions of the Faraday Society, 1962(58): 2200-2216.
[14] BARD A J, FAULKNER L R. Electrochemical methods fundamentals and applications [M]. 2nd Edition. Beijing: Chemical Industry Press, 2005: 110-153.
[15] MILCHEV A. Electrocrystallization—fundamentals of nucleation and growth [M]. New York: Kluwer Academic Publishers, 2002: 220-240
[16] ABYANEH M Y, FLEISCHMANN M. The electrocrystallization of nickel [J]. Journal of Electroanalytical Chemistry, 1981(119): 187-195.
[17] GOMEZ E, MULLER C, PROUD W G, VALLS E. Electro- deposition of nickel on vitreous carbon: Influence of potential on deposit morphology [J]. Journal of Applied Electrochemistry, 1992(22): 872-876.
[18] VAEZI M R, SADRNEZHAAD S K, NIKZAD L. Electrodeposition of Ni-SiC nano-composite coatings and evaluation of wear and corrosion resistance and electroplating characteristics [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 315(1/3): 176-182.
[19] BENEA L, BONORA P L, BORELLO A, MARTELLI S, WENGER F, PONTHIAUX P, GALLAND J. Composite electrodeposition to obtain nanostructured coating [J]. Journal of Electrochemical Society, 2001(148): 461-465.
[20] CAO Jing-qian. Effect of Cr(Ⅵ) on the deposition of Nickel [J]. Journal of Nanchang Institute of Aeronautical Technology, 1994(1): 14-19.
Corresponding author: TAN Cheng-yu; Tel: +86-731-8830270; Fax: +86-731-8876692; E-mail: tanchengyu@yahoo.com.cn
(Edited by LI Xiang-qun)