Influence of thermal ageing on oxidation performance and nanostructures of dry soot in diesel engine
来源期刊:中南大学学报(英文版)2021年第7期
论文作者:方嘉 孟忠伟 李健 张倩 黄俊峰 蒋渊 秦源 G G CHASE
文章页码:2206 - 2220
Key words:thermal ageing; oxidation performance; soot nanostructure; activation energy
Abstract: Diesel soot subjected to high exhaust temperature suffers from thermal ageing, which is difficult to be removed by regeneration process. Based on the thermogravimetric (TG) analysis and images by high resolution transmission electron microscope (HRTEM), effects of thermal ageing temperature, ageing time and oxygen concentration on oxidation characteristic of soot are investigated. The activation energy of soot increases with the increase of ageing temperature and oxygen concentration. The activation energy increases rapidly when the ageing time is less than 45 min, and then it keeps in a value of 157 kJ/mol when the ageing time is between 45 and 60 min. Compared to the soot without thermal ageing, the shape of ageing soot particles presents shorter diameter and more regular circle by observing soot nanostructure. With the increase of ageing temperature, ageing time and oxygen concentration, the more stable structure of “shell and core” is shown in the basic carbon. The soot has an increased fringe length, decreased tortuosity and separation distance after thermal ageing process, which leads to the deepening of the disorder degree of soot nanostructures and reduction of soot oxidation activity. Consequently, the thermal ageing process should be avoided in order to optimize the active regeneration strategy.
Cite this article as: MENG Zhong-wei, LI Jian, ZHANG Qian, HUANG Jun-feng, JIANG Yuan, QIN Yuan, G G CHASE4, FANG Jia. Influence of thermal ageing on oxidation performance and nanostructures of dry soot in diesel engine [J]. Journal of Central South University, 2021, 28(7): 2206-2220. DOI: https://doi.org/10.1007/s11771-021-4759-x.

J. Cent. South Univ. (2021) 28: 2206-2220
DOI: https://doi.org/10.1007/s11771-021-4759-x
MENG Zhong-wei(孟忠伟)1, 2, LI Jian(李健)3, ZHANG Qian(张倩)1, 2, HUANG Jun-feng(黄俊峰)1, 2,
JIANG Yuan(蒋渊)1, 2, QIN Yuan(秦源)1, 2, G G CHASE4, FANG Jia(方嘉)1, 2
1. Key Laboratory of Fluid and Power Machinery, Ministry of Education, Xihua University,Chengdu 610039, China;
2. Vehicle Measurement, Control and Safety Key Laboratory of Sichuan Province, Xihua University,Chengdu 610039, China;
3. Chengdu Qingzhouteji Environmental Protection Equipment Co., Ltd., Chengdu 610300, China;
4. Department of Chemical and Biomolecular Engineering, University of Akron, Akron,OH 44325-3906, United States
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract: Diesel soot subjected to high exhaust temperature suffers from thermal ageing, which is difficult to be removed by regeneration process. Based on the thermogravimetric (TG) analysis and images by high resolution transmission electron microscope (HRTEM), effects of thermal ageing temperature, ageing time and oxygen concentration on oxidation characteristic of soot are investigated. The activation energy of soot increases with the increase of ageing temperature and oxygen concentration. The activation energy increases rapidly when the ageing time is less than 45 min, and then it keeps in a value of 157 kJ/mol when the ageing time is between 45 and 60 min. Compared to the soot without thermal ageing, the shape of ageing soot particles presents shorter diameter and more regular circle by observing soot nanostructure. With the increase of ageing temperature, ageing time and oxygen concentration, the more stable structure of “shell and core” is shown in the basic carbon. The soot has an increased fringe length, decreased tortuosity and separation distance after thermal ageing process, which leads to the deepening of the disorder degree of soot nanostructures and reduction of soot oxidation activity. Consequently, the thermal ageing process should be avoided in order to optimize the active regeneration strategy.
Key words: thermal ageing; oxidation performance; soot nanostructure; activation energy
Cite this article as: MENG Zhong-wei, LI Jian, ZHANG Qian, HUANG Jun-feng, JIANG Yuan, QIN Yuan, G G CHASE4, FANG Jia. Influence of thermal ageing on oxidation performance and nanostructures of dry soot in diesel engine [J]. Journal of Central South University, 2021, 28(7): 2206-2220. DOI: https://doi.org/10.1007/s11771-021-4759-x.
1 Introduction
With the serious fuel consumption [1] and environmental problems [2], sustainable energy is applied to the internal combustion engines [3], but diesel engine is still the main power source for transportation [4] due to the good fuel economy, reliability and thermal efficiency [5]. However, the amounts of particulate matter (PM) emitted from diesel engines are much more than that emitted from gasoline engines, which limits the application of diesel engines [6]. Significantly, fine/micro PM exposure is harmful to human health [7]. In recent years, PM emission standards for diesel engines have become more and more stringent due to the growing concerns on public health [8] and environmental protection [9]. PM from the diesel exhaust gas can be called diesel soot [10], which can be effectively captured by diesel particulate filter (DPF) [11]. Nowadays, DPF with high trapping efficiency has been universally accepted as the most effective after treatment to satisfy the strict emission limits [12]. Among various kinds of DPF, wall-flow monolith DPF has a superiority in both filtration efficiency and pressure drop performance over the ceramic foams, open honeycomb structures, metallic wire-mesh or metal wools [13]. However, after long-time capturing, the accumulation of soot in the DPF causes the back pressure of engine to increase and the fuel efficiency to decrease [14, 15]. Consequently, in order to reduce gas flow resistance [16] and restore engine performance, the regeneration technology is applied to remove the accumulated PM [17]. In general, the methods for DPF regeneration process can be classified into active regeneration, passive regeneration and mixed passive-active regeneration [13]. The active regeneration can be realized by post fuel injection or additional heating device [18], such as the microwave assisted [19, 20], fuel burner and electrical heater [6], which leads to high temperature of the upstream in DPF (up to 550 °C or higher) [21] and the diminished fuel conversion efficiency [22]. The passive regeneration is carried out with the assistance of NO2 [23] or pre-catalyst [9], which greatly decreases regeneration temperature and time [9, 24]. The mixed active-passive regeneration is the combination of the above two regeneration methods [25], which is applicable only for catalyzed filters [23]. If the deposited soot particles are not cleared by regeneration in time, they will be affected by the high temperature exhaust, in which the thermal ageing occurs. Significantly, with the active regeneration method, the soot particles deposited in DPF are usually subjected to the thermal ageing of high exhaust temperature. Once soot particles suffer from thermal ageing process, it is difficult to eradicate them through the regeneration process, which leads to the service life time shortening and deterioration DPF. Thereby, it is necessary to study the effect of thermal ageing condition on the oxidation performance and nanostructure of soot.
Many experimental and numerical studies have been conducted to understand different ageing conditions on oxidation performance and microstructure of exhaust particle. GADDAM et al [26] presented an investigation for the influence of oxidation rates on three commercial carbon black (CB) nanostructures. They found that the changes of nanostructures corresponded to variations in the reaction activation energies. MENG et al [27] proposed that thermal oxidation treatment would remove hollow lattice and disordered structure of CB (Printex-U), which is helpful to lower carbon activation energy. However, the understanding on the effect of thermal ageing on the activation energy and nanostructure of diesel soot is still not clear. In addition, the temperature gradient distribution, regeneration frequency [28] and oxygen concentration [29] can influence both oxidation reaction performance and microstructure of the PM [30]. The process of soot oxidation can be evaluated by characteristic parameter (activation energy) [31] and nanostructure parameters (fringe length, separation distance, orientational ordering, tortuosity [32], and organization of the graphene layers [30]). OSCHATZ et al [33] provided a novel processing method on high resolution transmission electron microscopy (HRTEM) images to offer the information about the highly disordered nanostructure of porous carbide-derived carbon materials. They found that the size and density of parallel fringes and continuous domains increase with higher pyrolysis/chlorination temperatures. However, the results only focused on the effect of high temperature (above 700 °C) on nanostructure characterization of carbide-derived carbons, which did not involve the impact of microstructure and oxidation on soot treated under different lower temperatures corresponding the common diesel exhaust temperature (150-400 °C). WANG et al [34] studied the effect of oxygen concentration on soot oxidation in O2/CO2 atmosphere by thermogravimetric analyzer (TGA), and they concluded that the delays on the characteristic start, peak, and end temperatures of soot oxidation in O2/CO2 atmosphere were comparable to those of O2/N 2 atmosphere. Increase of O2 concentration promotes oxidation significantly and reduces the delays of the characteristic temperatures. CHONG et al [35] evaluated the effects of initial sample mass, proportion of volatile components of soluble organic fraction and oxygen concentration on the oxidation characteristics by TGA. These studies found the impact of oxygen concentration on soot oxidation performance, but did not reveal the influence of oxygen concentration on oxidation characteristic under the condition of thermal ageing. Furthermore, as mentioned above, the soot oxidation performance is closely correlated to its microstructure. YEHLIU et al [36] reported a new method to analyze the HRTEM images of carbon nanostructures [36], and compared the two HRTEM image analysis algorithms on fringe length, tortuosity and separation. The newly developed image analysis tool presented data in the form of a histogram and characteristic values (mean and median) [37]. WAL et al [38-40] studied the nanostructure of diesel soot using HRTEM image analysis, and observed that the oxidation of diesel soot could be strongly influenced by interior soot structure and morphology. WANG et al [41] reported that curvature quantification could be used to determine the distributions of segment frequency, segment length and angles between segments, and concluded that 28%-49% fringe presented curvature, and the most common inflection angles were in the range of 10°-20°. Those investigations provide an effective method to quantitatively evaluate the soot microstructure, and lay a solid foundation to investigate the oxidation performance and microstructure of diesel soot during the thermal ageing process.
To sum up, the mechanism of soot oxidation under the thermal ageing condition is involved in the limited literatures. In fact, effects of different thermal ageing conditions on soot oxidation necessitate exploration, which can lay a theoretical foundation for the active regeneration strategy of DPF. In order to optimize the control strategy of DPF active regeneration process, more detailed information about influence of thermal ageing temperature, ageing time and oxygen concentration on soot oxidation is needed. The oxidation performances and nanostructure of soot under different thermal ageing conditions are investigated by TGA and HRTEM, respectively. The identification of activation energy calculated by Arrhenius and Achar-Brindley-Sharp-Wendworth (ABSW) enables to describe the soot oxidation characteristics. The microscopic changes of the diesel soot under different ageing conditions can be reasonably evaluated by the nanostructure evolution imaged by HRTEM. The combination of oxidation characteristics and microscopic changes of soot is expected to describe the soot oxidation process comprehensively, which is conducive to the further improvement of the active regeneration strategy of DPF.
2 Description of experiments
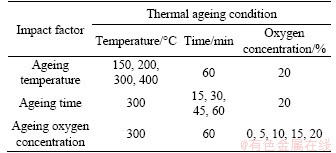
2.1 Materials and equipment
2.1.1 Sample collection
A common rail diesel engine (2.5 L, 85 kW, China IV) was applied to obtain diesel soot on an engine test bench. The specific specification of the engine is listed in Table 1. The metal screens were applied to collect diesel engine dry soot (DS) and the schematic diagram of filtration for DS is shown in Figure 1. The pure DS particles were obtained from the small aperture metal mesh when the operating condition was 1100 r/min and 100 N·m and the exhaust temperature was about 280 °C.
Table 1 Engine specification
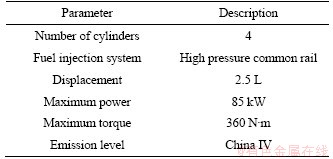

Figure 1 Schematic diagram of DS’s filtration
2.1.2 Thermogravimetric analysis
The TGA (TG209F3 from NETZSCH, Germany) with recording software was applied to measure the oxidation rates of DS, and the main parameters are listed in Table 2. The mass loss of samples under a wide temperature range and the controlled air atmosphere were measured and recorded by the TGA. Experiments were conducted with 2 mg DS for each test. The samples were pretreated under different thermal ageing conditions as shown in Table 3, and then oxidized under the same experimental condition. The temperature interval, keeping time, gas flow rate, heating rate and oxygen concentration were 45-800 °C, 10 min,100 mL/min, 30 °C/min and 0-20%, respectively.
Table 2 Main parameters of TG209F3
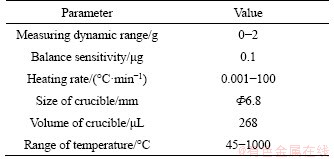
Table 3 Detail of different thermal ageing conditions for diesel soot
2.1.3 High resolution transmission electron microscopy (HRTEM)
HRTEM instrument (Tecnai G2 F20, American) was applied to obtain the microstructure images of diesel soot after thermal ageing. For HRTEM imaging, diesel soot was deposited on a lacey C/Cu grid by depositing a drop of the soot suspension, which was created by sonication in ethanol. YEHLIU et al [36] found out that fringe length and tortuosity could be processed through some steps, mainly including selecting the regions of interest, improving contrast, Gaussian filter, binarization, perform morphological opening and closing, skeletonizing and so on. Fringe length is the physical extent of the atomic carbon layer planes as seen in the HRTEM image. Larger length is corresponding to a higher level of organization, so that the material is more resemble to graphitic structure. Tortuosity is defined as the ratio of the actual fringe length to the shortest distance between the endpoints of carbon segment. The schematic diagram of DS microstructure is displayed in Figure 2, C=L/D, where C is the tortuosity, L is the fringe length, D is the end point distance. ImageJ software was applied to obtain fringe length and tortuosity from the HRTEM images. Similarly, Gatan Digital Micrograph was also used to determine the separation distance.

Figure 2 Schematic diagram of DS microstructure
2.2 Data analysis
The soot oxidation kinetics are analyzed by Arrhenius and ABSW methods, respectively. The conversion fraction (α) of soot oxidation can be defined as:
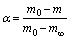 (1)
(1)
where m0, m and m∞ are the original sample mass, remaining sample mass and final sample mass, respectively. The above parameters are automatically recorded by the TGA.
Since oxidation of soot by oxygen is a solid-gas reaction, the chemical reaction mainly occurs on the active sites of the surface of soot particles. The relative reaction rate γ′is expressed as [23, 24, 42]:
 (2)
(2)
where A′, E, R, T, n, Sg and PO2represent the pre-exponential factor, activation energy, gas constant, absolute temperature, reaction order, specific surface area of carbon black and the oxygen partial pressure, respectively.
Method 1: Arrhenius method
The natural logarithm of Eq. (2) is employed by the Arrhenius method. Thus, the result can be expressed as:
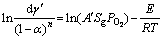 (3)
(3)
where 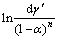 and 1/T are linearly related, then E/R is the slope of fitting line, ln(A′SgPO2) is the interception, and E can be calculated by Arrhenius method.
and 1/T are linearly related, then E/R is the slope of fitting line, ln(A′SgPO2) is the interception, and E can be calculated by Arrhenius method.
Method 2: ABSW method
In the method of differential ABSW, separating variables in Eq. (2) are prior to apply the natural log to obtain the expression:
 (4)
(4)
where heating rate β is also expressed as β=dT/dt. From Eq. (4), the left side of this equation has a linear relation with 1/T, E and A′ can also be calculated by the slope and vertical intercept of the matched curve, respectively [25]. To facilitate comparison of activation energy E of different ageing soot particles, both Arrhenius and ABSW methods are applied.
3 Results and analysis
3.1 Thermal ageing temperature effect on soot oxidation performance
Figure 3 shows the TG curves of soot under different ageing temperatures with constant thermal ageing time and oxygen concentration as indicated in Table 3. The TG curve of soot without thermal ageing can be divided into two stages. The first stage ranges from 0 to 200 °C, in which the soot particles lose weight slowly and the mass loss drops down by 5%. It is mainly caused by water evaporation and condensed hydrocarbons [21]. The soot oxidation reaction happens at the second stage which ranges from 200 to 800 °C. The TG curves go down obviously with the increase of temperature because of the fully contact between soot particles and oxygen. In comparison with the soot without thermal ageing (marked as “no treatment”), the TG curves of the ageing soot do not have the first stage because the moisture in the soot particles evaporates in the thermal ageing process. The mass loss occurs mainly in the second stage from the TG curves of the ageing soot samples. The initiation oxidation temperature of the ageing soot gradually increases with rising temperature, so that the TG curves gradually shift to high temperature region. The deviation trend is more obvious with the increase of ageing temperature. It may be explained that the ageing soot has a more stable structure which makes the soot less prone to oxidize, and the higher the ageing temperature is, the more obvious the change of the soot structure is. The TG curves tend to overlap in the temperature range between 550 and 580 °C whether the soot is subjected to thermal ageing or not. In summary, the thermal ageing temperature has great influence on the earlier stage of oxidation reaction but little influence on the high temperature stage.
The activation energy E calculated by Arrhenius and ABSW methods of soot particles under different ageing temperatures are shown in Figure 4. The E of thermal ageing soot increases by about 42-115 kJ/mol compared with the soot without thermal ageing (73 kJ/mol). E of the ageing soot increases slowly when the ageing temperature is less than 200 °C, while increases sharply when the ageing temperature ranges from 200 to 400 °C. One reason may be that the easy reactive and volatile components of soot are oxidized during thermal ageing process and the remaining refractory components increase. Meanwhile, the consumption of the easily reactive and volatile components is different under various ageing temperatures, which reduces with ageing temperature. The amount of the unreactive and less volatile materials increases. Therefore, E accordingly increases with the increase of ageing temperature. Another reason may be that the surfaces of the basic carbon form different degrees of graphitization when soot particles are under high temperature thermal ageing [26]. This leads the soot oxidation activity to reduce and E of soot to increase as thermal ageing temperature increases. In addition, the soot particles can be obviously oxidized when the ageing temperature exceeds 400 °C in the thermal ageing process, so that the ageing temperatures in the subsequent experiments are below 400 °C in order to obtain the expected ageing soot samples.
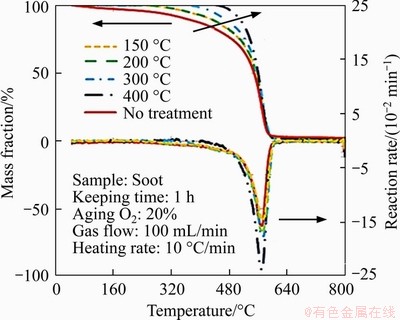
Figure 3 TG curves of soot particles under different ageing temperatures
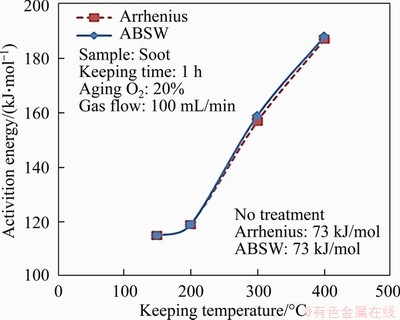
Figure 4 Activation energy E of soot particles under different ageing temperatures
3.2 Effect of thermal ageing time on soot oxidation performance
Figure 5 shows the TG curves of soot with different thermal ageing time with constant thermal ageing temperature and oxygen concentration as indicated in Table 3. From Figure 5, the TG curves of soot with different ageing time shift to high temperature region compared with the soot without thermal ageing process. Moreover, the curves also gradually shift to higher temperature region with the increase of thermal ageing time. According to the TG curves, it can be concluded that the ageing time has a greater impact on the earlier stage of soot oxidation than the later oxidation stage. The longer the ageing time is, the more stable the soot microstructure is. Compared with Figure 3, the tendency of the ageing soot shifting to the high temperature area is more obvious than that of soot particles suffering from thermal ageing under different temperatures. On the contrary, the tendency of soot shifting to the high temperature region is relatively weak compared with that subjected to thermal ageing for different ageing times. In summary, thermal ageing temperature has more impact on oxidation performance of soot than thermal ageing time.
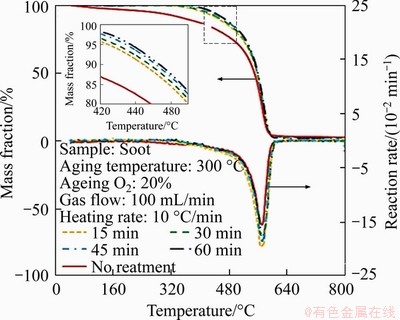
Figure 5 TG curves of soot with different ageing time
Figure 6 shows the E of soot under different thermal ageing times, which is calculated by Arrhenius and ABSW methods. As seen, E of soot increased by 67-87 kJ/mol with different thermal ageing time, compared with the soot without thermal ageing (73 kJ/mol). When the ageing time is less than 45 min, E of soot increases approximately linearly with the increase of ageing time. The E of soot with thermal ageing tends to be stable when the ageing time is from 45 to 60 min. This could be explained that the easily oxidized and volatile components have been reacted with the increase of the thermal ageing time, which results in that the rest of diesel soot after long time thermal ageing is difficult to oxidize. When thermal ageing of soot is conducted under the same temperature, the proportion of the inert components and E of soot increase firstly with the increase of ageing time. The influence of inert components on E is limited when soot is heated at the same temperature after enough thermal ageing time. Therefore, E tends to remain constantly after the ageing time increasing to a certain extent. In addition, the microstructure of soot changes when the soot is under different ageing time. For instance, the fringe length becomes longer, and the carbon layer is denser, which may cause the E of soot increase under different thermal ageing times, and the results will be discussed in Section 3.4.
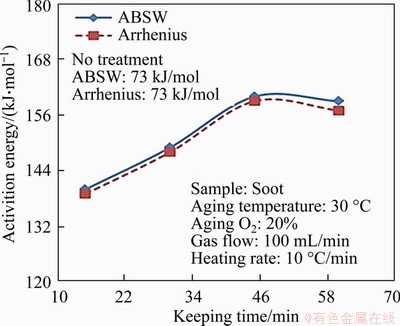
Figure 6 Activation energy E of soot with different ageing time
3.3 Effect of thermal ageing oxygen concentration on soot oxidation performance
Figure 7 shows TG curves of thermal ageing soot under different oxygen concentrations with constant thermal ageing time and ageing temperature as indicated in Table 3. All the TG curves of soot with thermal ageing or not are divided into two stages. The decline of the first stage is mainly due to water evaporation and the oxidation of substance which is easy to be oxidized. The other stage is mostly the oxidation of soot, which leads to the weight loss of diesel soot. Because the thermal ageing is carried out in aerobic atmosphere (except 0% O2 condition), the oxidative substances in soot are oxidized in advance, which leads to the first stage of TG curves of soot vanishes under different oxygen concentrations during the thermal ageing process. Compared with the soot without thermal ageing process, the TG curves of the soot after thermal ageing process shift to higher temperatures. Compared with the ageing soot in anaerobic atmosphere (N2 condition), the tendency of soot in aerobic atmosphere shifting to high temperature region is more obvious, which indicates that there is easily oxidized substance existing in diesel soot besides few water and volatile substance. The oxidation of the easily reactive substances in the soot particles happens in advance under the thermal ageing with the oxygen condition, which maybe leads the TG curves to shift to the high temperature zone. In summary, the oxidative activity of soot is reduced by thermal ageing under different ageing oxygen concentrations. The TG curves of soot gradually tend to overlap in the late high temperature stage of oxidation regardless of the ageing oxygen concentration, and have the similar oxidation characteristic.

Figure 7 TG curves of soot under different ageing oxygen concentrations
Oxidation kinetics is used to analyze the TG curves of thermal ageing soot under different oxygen concentrations and the results are shown in Figure 8. It can be seen that the E of thermal ageing soot increases regardless of the oxygen concentration. Compared with the soot without thermal ageing process, E of the soot after thermal ageing under anaerobic atmosphere (N2 condition) increases by 46 kJ/mol approximately, and E of thermal ageing soot under aerobic atmosphere (oxygen concentration from 5% to 20%) increases largely to 82-89 kJ/mol. However, E of soot particles after thermal ageing process under the aerobic atmosphere are almost constantly 154-164 kJ/mol, and has little significant difference with the change of ageing oxygen concentrations. The main reason is that the oxidative substance of soot particles reacts with oxygen during the thermal ageing process under aerobic atmosphere. However, when soot particles are subjected to thermal ageing under anaerobic atmosphere (N2 condition, 0% O2), only water and volatile matter in soot are evaporated. This is the reason that E of soot particles under aerobic conditions is higher than that of soot particles after thermal ageing without oxygen. Finally, the soot particles suffer the thermal ageing process no matter whether under aerobic conditions, the surface of the soot is affected by the high temperature gas and forms a densely carbon layer, which causes the graphitization of the surface. Therefore, E of the soot particle after thermal ageing process increases compared with the soot without thermal ageing process.
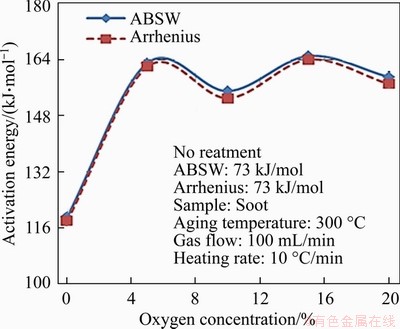
Figure 8 Activation energy of soot under different ageing oxygen concentrations
3.4 Nanostructures of soot under different thermal ageing conditions
According to the results of all TG analyses, thermal ageing has a certain negative effect on the oxidation of soot, and reduces the oxidation activity of soot. E of soot particles increases when the ageing temperature, ageing time and ageing oxygen concentration increase. From Figures 4, 6 and 8, the influence of ageing temperature on the oxidation of soot is larger than ageing time and ageing oxygen concentration. Meanwhile, the effect of ageing time is larger than ageing oxygen concentration under the aerobic atmosphere.
In order to further investigate the influence of thermal ageing on the oxidation characteristic of soot, HRTEM images are obtained to analyze the change of nanostructure of soot after thermal ageing under different conditions. From Figure 9, the nanostructure is consisted of the ordered “shell” of outer and the disordered “core” of inner [43, 44]. Compared with Figure 9(a), the diameters of the soot particles after different ageing conditions are significant smaller, and the shape is more regular circle, as shown in Figures 9(b)-(e). Comparing Figures 9(b)-(e), when the thermal ageing temperature is higher and the ageing time is longer under aerobic atmosphere, the diameters of soot particles are smaller, and the outer layer structure is also denser and the area of core is smaller. In other words, the reactive or unstable components of soot reacted or evaporated during the process of thermal ageing process, the core of basic carbon forms a more stable shell. The increase of the graphitization degree of the external structure induces the soot structure more stable [45], which leads to decreased oxidation activity and increased activation energy. The results of nanostructure change also are in accordance with the results of TG experiments.
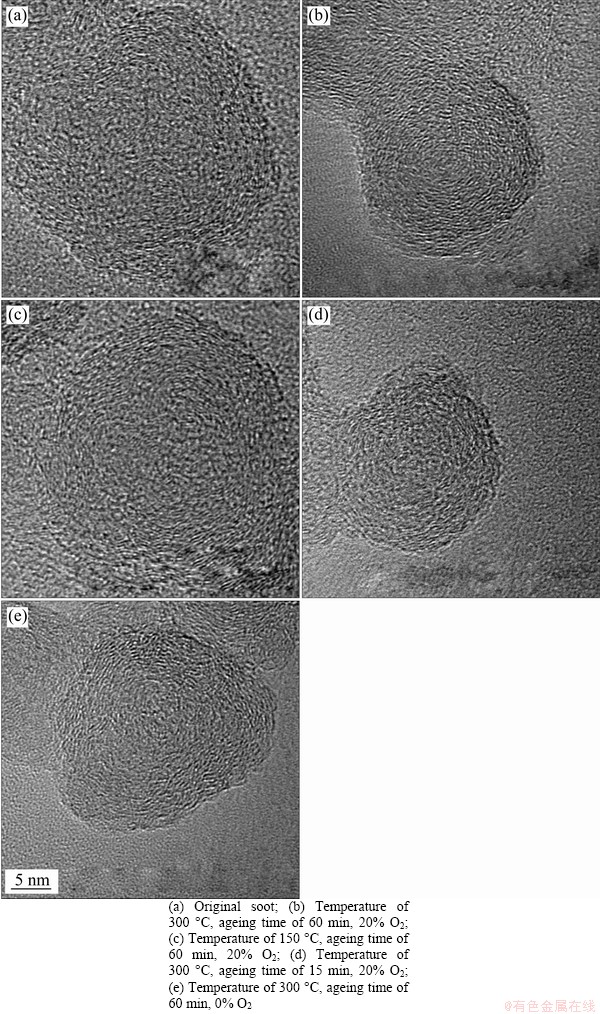
Figure 9 Microstructures of diesel soot under different thermal ageing conditions:
The software of Image J and digital micrograph are applied to analyze the nanostructure of soot after different thermal ageing conditions. The parameters of nanostructure of soot, including fringe length, tortuosity and separation distance are shown in Figures 10-12, respectively. Figure 10 is the distribution diagram of fringe length of soot after thermal ageing under various conditions and the distribution of microcrystalline length is always unimodal and mainly distributes between 0 and 0.5 nm. From Figures 10(a) and (b), the proportion of the fringe length of the soot after thermal ageing process increases significantly in the region of greater than 0.5 nm, which indicates that the fringe length of soot after thermal ageing process increases. Comparing Figure 10(c) with Figure 10(b), the fringe length increases in a little degree with the increase of ageing temperature in the region of greater than 1.0 nm. Comparing Figure 10(d) with Figure 10(b), the fringe length is almost constantly when the ageing time is less than 15 min, but also gradually increases when ageing time is 60 min. Similarly, from Figures 10(a), (b) and (e), the fringe length distribution of the soot after thermal ageing without oxygen (Figure 10(e)) is similar to that of soot after thermal ageing in oxygen condition (Figure 10(b)), while the percent of fringe length of the ageing soot (Figure 10(e)) increases a little compared with that of the soot without thermal ageing (Figure 10(a)) in the region of greater than 1.5 nm. In summary, both the thermal ageing temperature and time can obviously affect the fringe length of diesel soot after thermal ageing process, while the oxygen concentration does not present great influence on fringe length of diesel soot. Furthermore, larger fringe length trends to present higher E of soot.
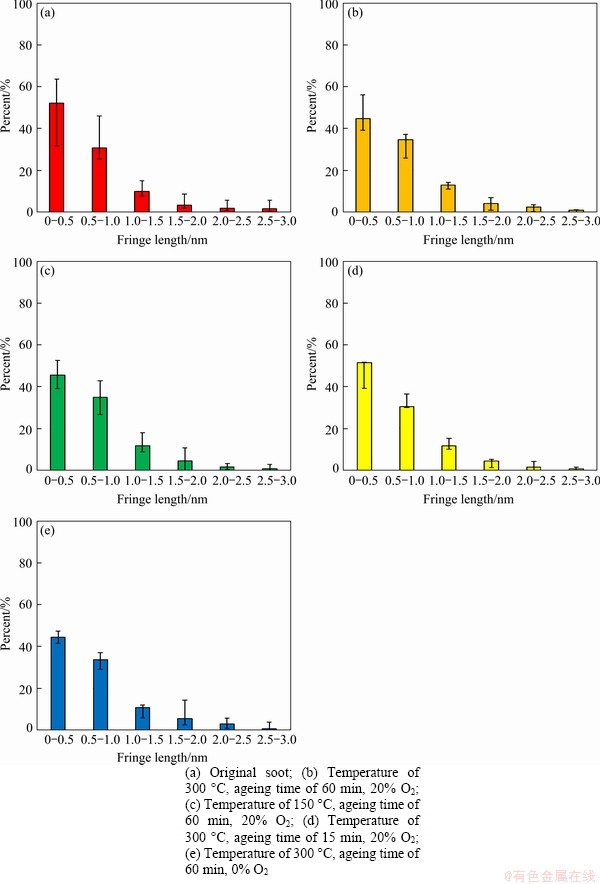
Figure 10 Fringe length of diesel soot under different thermal ageing conditions:
Figure 11 shows the distribution of tortuosity of the soot after thermal ageing under various conditions. The results display that the distribution of tortuosity is also unimodal mainly ranging from 1 to 2, the peak value is always between 1 and 1.2. From Figures 11(a) and (b), the proportion of the tortuosity of soot in the region of 1-1.2 significantly increases compared with the soot after thermal ageing process. Comparing Figures 11(b) and (c) with Figure 11(a), when ageing temperature is lower than 150 °C, the distribution of tortuosity almost keeps constant regardless of the low temperature thermal ageing. However, when the ageing temperature is higher, the ratio of the distribution of tortuosity in smaller value region (1-1.2) increases obviously, which indicates that the increasing ageing temperature (larger than 150 °C) leads to the decrease of tortuosity. Comparing Figures 11(d) to 11(b), the increasing ageing time leads to the proportion of tortuosity in smaller value region increasing slowly. From Figures 11(e) and (b), compared with the soot without thermal ageing, the tortuosity of soot after thermal ageing under anaerobic atmosphere increases obviously, while soot under aerobic atmosphere thermal ageing process tends to decrease the tortuosity. Besides, smaller tortuosity trended to present higher E of soot. The fringe length is an important parameter, and the third one is the separation distance of soot.
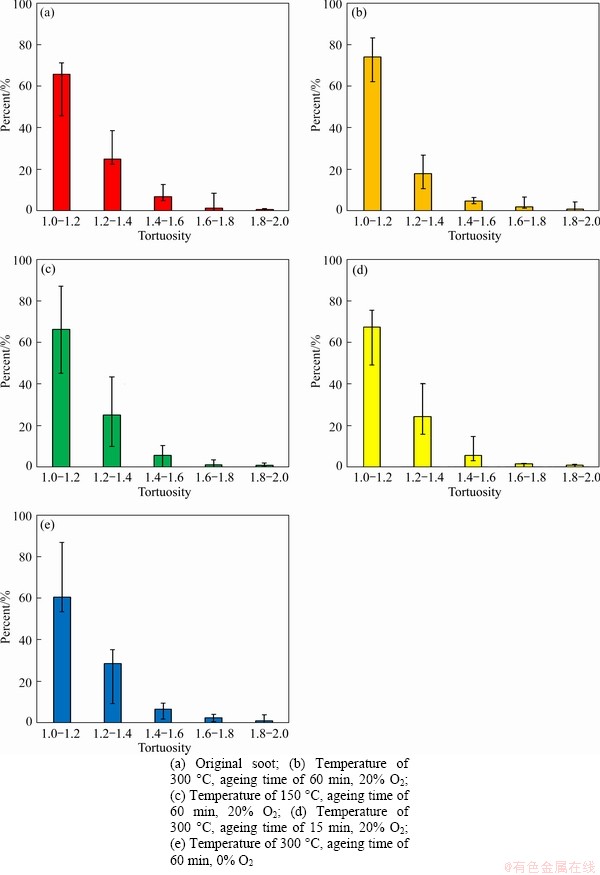
Figure 11 Tortuosity of diesel soot under different thermal ageing conditions:
Figure 12 shows the distribution diagrams of separation distance of ageing soot under different thermal ageing conditions. The distributions of separation distance of soot are mainly between 0.3 and 0.6 nm regardless of the effect of thermal ageing process. Compared with the soot without thermal ageing, the proportion of the distribution of the soot after thermal ageing increases in the smaller value area (less than 0.4 nm) and decreases in larger value region obviously, which indicates that thermal ageing leads to the increase of separation distance generally. Comparing Figures 12(b)-(d) with Figure 12(a), the ratio of separation distance in smaller value region (less than 0.4 nm) continuously increases with the increase of ageing temperature and ageing time. Similarly, the ratio of separation distance in smaller value area (less than 0.4 nm) increases in both anaerobic and aerobic conditions in both Figures 12(e) and (b). Moreover, the proportion of separation distance in smaller value region is higher with the increase of oxygen concentration. In summary, all the thermal ageing temperature, time and oxygen concentration can obviously affect the separation distance, and the higher value of thermal ageing parameters tends to present smaller separation distance of soot.
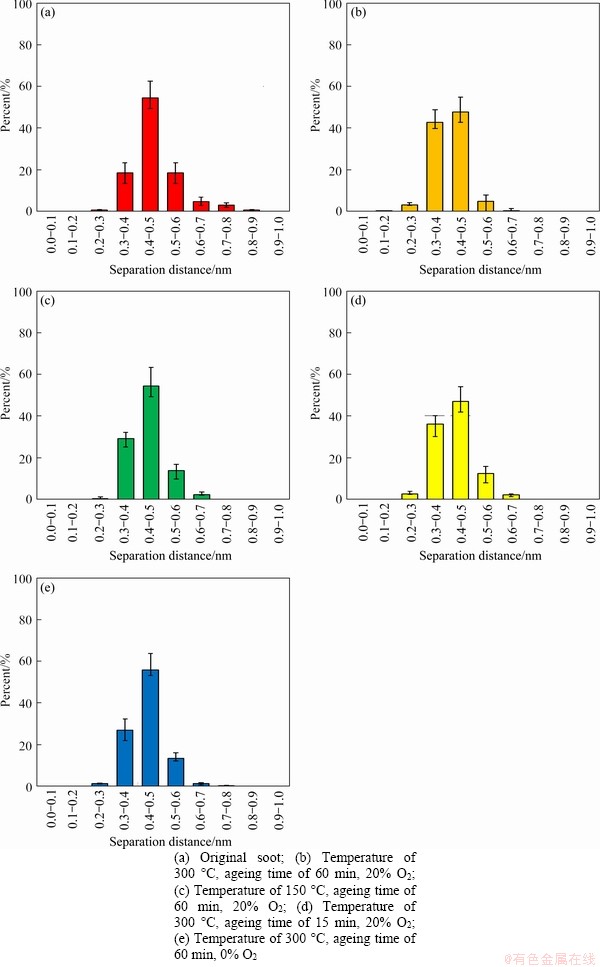
Figure 12 Distribution diagrams of separation distance of ageing soot under different thermal ageing conditions:
The nanostructure parameters of soot with thermal ageing process under different conditions are averaged and the results are listed in Table 4. Comparing the two samples A and B in Table 4, the fringe length increases, and the tortuosity and separation distance all decrease as thermal ageing temperature, ageing time and ageing oxygen concentration concurrently increase. The main reason is the unordered structure in carbon granule reduces, and the ratio of the active carbon atom and oxidative activity all gradually decrease, which leads to the decrease of E.
Comparing samples C and D with B, with the increase of ageing temperature and ageing time, the tortuosity and separation distance decrease gradually,and the fringe length increases which is caused by the decreasing oxidation activity of the basic carbon and increasing E. However, compared with samples E with B, it can be revealed that fringe length, tortuosity and separation distance all gradually decrease with the increase of ageing oxygen concentration. The decreasing of fringe length is helpful to increase oxidative activity, but the tortuosity and separation distance are acted as the negative affection. Meanwhile, from the E of samples B and E, it could be found that the variation of fringe structure inside carbon atom is caused by the interaction of fringe length, tortuosity and separation distance. The effect of tortuosity and separation distance has more influence on the soot oxidative activity than the effect of fringe length.
Table 4 Average values of nanostructure parameters of soot with different ageing conditions
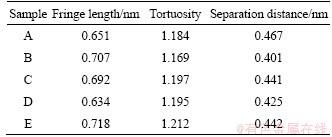
The E of samples C and E are all larger than that of A. However, compared samples C and E with A, fringe length of samples C and E increases, and separation distance varies as opposite direction.The reason can be that the impact of fringe length and separation on the oxidation characteristic of carbon atom is larger than that of tortuosity. The separation distance is the primary impact factor of soot oxidation performance compared with fringe length and tortuosity.
In conclusion, thermal ageing process affects the fringe structure of carbon granule, which leads to the variation of E. Different ageing temperatures, ageing times and oxygen concentrations have different effects on the microstructure of soot particles. The fringe structure (fringe length, tortuosity and separation distance) has synergetic effect on the soot oxidation performance. The results show that the separation distance acts as the chief impact factor compared with the other two factors.
4 Conclusions
The influences of thermal ageing temperature, ageing time and ageing oxygen concentration on the oxidation performance and the nanostructures of soot are investigated by the TGA and HRTEM. The conclusions can be obtained from the experimental results.
1) The activation energy of diesel soot increases after thermal ageing process, which indicates that thermal ageing process has a negative effect on the soot oxidation characteristic as follows.
2) The activation energy of thermal ageing soot first increases slowly when ageing temperature is higher than 200 °C, and it increases sharply when the ageing temperature is higher than 200 °C. The activation energy of diesel soot is approximately linear when the ageing time is less than 45 min, and it almost keeps constantly when the ageing time is between 45 and 60 min. The activation energy of diesel soot after thermal ageing under aerobic condition is higher than that of soot under anaerobic condition. However, the variation of thermal ageing oxygen concentration (ranged from 5% to 20%) has no obvious effect on the activation energy of soot.
3) Compared with diesel soot without thermal ageing, the diameters of diesel soot after thermal ageing are significantly reduced, and the shape presents as more regular circles. The outer shell of the basic carbon is more dense, while the inner core is smaller and forms more stable structure of “shell and core” when the thermal ageing temperature, aging time and oxygen concentration are increased.
4) In general, the fringe length increases, while the tortuosity and separation distance decreases after the soot thermal ageing process, which leads to the increase of the disorder degree of particle nanostructures and decrease of soot oxidation activity.
5) Different thermal ageing conditions have various effects on the nanostructure of diesel soot. The separation distance is acting as the primary influence factor compared with the soot fringe length and tortuosity.
Between the intervals of two active regeneration processes of DPF, soot particles will be aging at high temperature. The investigation on the influence of thermal aging on soot oxidation characteristics is beneficial to accurately master the soot oxidation during active regeneration process of DPF, and provides an experimental reference for the formulation of regeneration strategy for DPF.
Contributors
MENG Zhong-wei provided the idea of the study, and led the research activity planning and execution. LI Jian conducted all the experiments and drafted the manuscript. ZHANG Qian, HUANG Jun-feng, JIANG Yuan and QIN Yuan conducted the experiments, and analyzed the experimental data. CHASE G G revised the manuscript. FANG Jia revised the manuscript and was responsible for the submission and reply of the manuscript as the corresponding author. All the authors replied to reviewers’ comments and revised the final version.
Conflict of interest
MENG Zhong-wei, LI Jian, ZHANG Qian, HUANG Jun-feng, JIANG Yuan, QIN Yuan, G G CHASE, FANG Jia declare that they have no conflict of interest.
References
[1] CHU Hua-qiang, XIANG Long-kai, NIE Xiao-kang, YA Yu-chen, GU Ming-yan, E Jia-qiang. Laminar burning velocity and pollutant emissions of the gasoline components and its surrogate fuels: A review [J]. Fuel, 2020, 269: 117451. DOI: 10.1016/j.fuel.2020.117451.
[2] HAN Wei-wei, ZHOU Yong, ZHU Ting, CHU Hua-qiang. Combustion synthesis of defect-rich carbon nanotubes as anodes for sodium-ion batteries [J]. Applied Surface Science, 2020, 520: 146317. DOI: 10.1016/j.apsusc.2020.146317.
[3] LI Yuan-xu, NING Zhi, YAN Jun-hao, LEE T H, LEE C F. Experimental investigation on combustion and unregulated emission characteristics of butanol-isomer/gasoline blends [J]. Journal of Central South University, 2019, 26(8): 2244-2258. DOI: 10.1007/s11771-019-4170-z.
[4] ZHANG Gui-ju, E Jia-qiang, ZUO Qing-song, GONG Jin-ke, ZUO Wei, YUAN Wen-hua. Numerical simulation on trapping efficiency of steady filtration process in diesel particulate filter and its experimental verification [J]. Journal of Central South University, 2015, 22(11): 4456-4466. DOI: 10.1007/s11771-015-2993-9.
[5] ZHANG Qian, FANG Jia, MENG Zhong-wei, CHEN Chen, QIN Zi-han. Thermogravimetric analysis of soot combustion in the presence of ash and soluble organic fraction [J]. RSC Advances, 2020, 10(55): 33436-33443. DOI: 10.1039/ d0ra06384c.
[6] MENG Zhong-wei, CHEN Chao, LI Jian-song, FANG Jia, TAN Jie, QIN Yuan, JIANG Yuan, QIN Zi-han, BAI Wei-lian, LIANG Kun. Particle emission characteristics of DPF regeneration from DPF regeneration bench and diesel engine bench measurements [J]. Fuel, 2020, 262: 116589. DOI: 10.1016/j.fuel.2019.116589.
[7] WANG Fei-fei, ZHANG En-shi, XU Xin-hua, WANG Jin-bo, MI Jian-chun. Particle deposition in ventilation duct with convex or concave wall cavity [J]. Journal of Central South University, 2018, 25(11): 2601-2614. DOI: 10.1007/s11771-018-3939-9.
[8] SHI Yun-xi, CAI Yi-xi, LI Xiao-hua, JI Liang, CHEN Yi, WANG Wei-kai. Evolution of diesel particulate physicochemical properties using nonthermal plasma [J]. Fuel, 2019, 253: 1292-1299. DOI: 10.1016/j.fuel.2019.05.10.
[9] FANG Jia, MENG Zhong-wei, LI Jian-song, DU Yu-heng, QIN Yuan, JIANG Yuan, BAI Wei-lian, CHASE G G. The effect of operating parameters on regeneration characteristics and particulate emission characteristics of diesel particulate filters [J]. Applied Thermal Engineering, 2019, 148: 860-867. DOI: 10.1016/j.applthermaleng.2018.11.066.
[10] DU Yu-heng, MENG Zhong-wei, FANG Jia, QIN Yuan, JIANG Yuan, LI Shuang, LI Jian-song, CHEN Chao, BAI Wei-lian. Characterization of soot deposition and oxidation process on catalytic diesel particulate filter with ash loading through an optimized visualized method [J]. Fuel, 2019, 243: 251-261. DOI: 10.1016/j.fuel.2019.01.103.
[11] BUONO D, SENATORE A, PRATI M V. Particulate filter behaviour of a diesel engine fueled with biodiesel [J]. Applied Thermal Engineering, 2012, 49: 147-153. DOI: 10.1016/ j.applthermaleng.2011.08.019.
[12] SHI Yun-xi, CAI Yi-xi, FAN Run-lin, CUI Ying-xin, CHEN Yi, JI Liang. Characterization of soot inside a diesel particulate filter during a nonthermal plasma promoted regeneration step [J]. Applied Thermal Engineering, 2019, 150: 612-619. DOI: 10.1016/j.applthermaleng.2019.01.015.
[13] ROSSOMANDO B, ARSIE I, MELONI E, PALMA V, PIANESE C. Experimental testing of a low temperature regenerating catalytic DPF at the exhaust of a light-duty diesel engine [C]// WCX World Congress Experience, 2018-01-0351. DOI: 10.4271/2018-01-0351.
[14] MENG Zhong-wei, LI Jian-song, FANG Jia, TAN Jie, QIN Yuan, JIANG Yuan, QIN Zi-han, BAI Wei-lian, LIANG Kun. Experimental study on regeneration performance and particle emission characteristics of DPF with different inlet transition sections lengths [J]. Fuel, 2020, 262: 116487. DOI: 10.1016/j.fuel.2019.11648.
[15] FANG Jia, QIN Zi-han, MENG Zhong-wei, JIANG Yuan, LIU Jing, ZHANG Qian, TAN Jie. Performance of diesel soot oxidation in the presence of ash species [J]. Energy & Fuels, 2020, 34(2): 2185-2192. DOI: 10.1021/acs.energyfuels. 9b03085.
[16] FANG Jia, MENG Zhong-wei, LI Jian, PU Yun-fei, DU Yu-heng, LI Jian-song, JIN Zhao-xiang, CHEN Chao, CHASE G G. The influence of ash on soot deposition and regeneration processes in diesel particular filter [J]. Applied Thermal Engineering, 2017, 124: 633-640. DOI: 10.1016/ j.applthermaleng.2017.06.076.
[17] FANG Jia, ZHANG Qian, MENG Zhong-wei, LUO Yan, OU Juan, DU Yu-heng, ZHANG Zhi-lin. Effects of ash composition and ash stack heights on soot deposition and oxidation processes in catalytic diesel particulate filter [J]. Journal of the Energy Institute, 2020, 93: 1942-1950. DOI: 10.1016/j.joei.2020.04.009.
[18] LIU Jun-heng, WANG Le-jian, SUN Ping, WANG Pan, LI Yu-qiang, MA Hong-jie, WU Peng-cheng, LIU Zeng-guang. Effects of iron-based fuel borne catalyst addition on microstructure, element composition and oxidation activity of diesel exhaust particles [J]. Fuel, 2020, 270: 117597. DOI: 10.1016/j.fuel.2020.117597.
[19] E Jia-qiang, ZHAO Xiao-huan, XIE Long-fu, ZHANG Bin, CHEN Jing-wei, ZUO Qing-song, HAN Dan-dan, HU Wen-yu, ZHANG Zhi-qing. Performance enhancement of microwave assisted regeneration in a wall-flow diesel particulate filter based on field synergy theory [J]. Energy, 2019, 169: 719-729. DOI:10.1016/j.energy.2018.12.086.
[20] PALMA V, MELONI E. Microwave assisted regeneration of a catalytic diesel soot trap [J]. Fuel, 2016, 181: 421-429. DOI: 10.1016/j.fuel.2016.05.016.
[21] FANG Jia, SHI Rong, MENG Zhong-wei, JIANG Yuan, QIN Zi-han, ZHANG Qian, QIN Yuan, TAN Jie, BAI Wei-lian. The interaction effect of catalyst and ash on diesel soot oxidation by thermogravimetric analysis [J]. Fuel, 2019, 258: 116151. DOI: 10.1016/j.fuel.2019.116151.
[22] LIATI A, EGGENSCHWILER P D, SCHREIBER D, ZELENAY V, AMMANN M. Variations in diesel soot reactivity along the exhaust after-treatment system, based on the morphology and nanostructure of primary soot particles [J]. Combustion and Flame, 2013, 160(3): 671-681. DOI: 10.1016/j.combustflame.2012.10.024.
[23] ROSSOMANDO B, ARSIE I, MELONI E, PALMA V, PIANESE C. Experimental test on the feasibility of passive regeneration in a catalytic dpf at the exhaust of a light-duty diesel engine [C]// 14th International Conference on Engines & Vehicles, 2019-24-0045. DOI: 10.4271/2019-24-0045.
[24] E Jia-qiang, ZUO Wei, GAO Jun-xu, PENG Qing-guo, ZHANG Zhi-qing, HIEU P M. Effect analysis on pressure drop of the continuous regeneration-diesel particulate filter based on NO2 assisted regeneration [J]. Applied Thermal Engineering, 2016, 100: 356-366. DOI: 10.1016/ j.applthermaleng.2016.02.031.
[25] MELONI E, PALMA V, VAIANO V. Optimized microwave susceptible catalytic diesel soot trap [J]. Fuel, 2017, 205: 142-152. DOI: 10.1016/j.fuel.2017.05.074.
[26] GADDAM C K, WAL R V, CHEN Xu, YEZERETS A, KAMASAMUDRAM K. Reconciliation of carbon oxidation rates and activation energies based on changing nanostructure [J]. Carbon, 2016, 98: 545-556. DOI: 10.1016/j.carbon. 2015.11.035.
[27] MENG Zhong-wei, YANG Dong, YAN Y. Study of carbon black oxidation behavior under different heating rates [J]. Journal of Thermal Analysis and Calorimetry, 2014, 118(1): 551-559. DOI: 10.1007/s10973-014-4020-z.
[28] DENG Yuan-wang, CUI Jin-hui, E Jia-qiang, ZHANG Bin, ZHAO Xiao-huan, ZHANG Zhi-qing, HAN Dan-dan. Investigations on the temperature distribution of the diesel particulate filter in the thermal regeneration process and its field synergy analysis [J]. Applied Thermal Engineering, 2017, 123: 92-102. DOI: 10.1016/j.applthermaleng.2017.05.072.
[29] BREDIN A, LARCHER A V, MULLINS B J. Thermogravimetric analysis of carbon black and engine soot—Towards a more robust oil analysis method [J]. Tribology International, 2011, 44(12): 1642-1650. DOI: 10.1016/j.triboint.2011.06.002.
[30] YEHLIU K, WAL R V, ARMAS O, BOEHMAN A L. Impact of fuel formulation on the nanostructure and reactivity of diesel soot [J]. Combustion and Flame, 2012, 159(12): 3597-3606. DOI: 10.1016/j.combustflame.2012.07.004.
[31] RAJ A, YANG S Y, CHA D, TAYOUO R, CHUNG S H. Structural effects on the oxidation of soot particles by O2: Experimental and theoretical study [J]. Combustion and Flame, 2013, 160(9): 1812-1826. DOI: 10.1016/ j.combustflame.2013.03.010.
[32] JARAMILLO I C, GADDAM C K, WAL R V, HUANG C H, LEVINTHAL J D, LIGHTY J S. Soot oxidation kinetics under pressurized conditions [J]. Combustion and Flame, 2014, 161(11): 2951-2965. DOI: 10.1016/j.combustflame.2014. 04.016.
[33] OSCHATZ M, PRE P, DORFLER S, NICKEL W, BEAUNIER P, ROUZAUD J-N, FISCHER C, BRUNNER E, KASKEL S. Nanostructure characterization of carbide-derived carbons by morphological analysis of transmission electron microscopy images combined with physisorption and Raman spectroscopy [J]. Carbon, 2016, 105: 314-322. DOI: 10.1016/j.carbon.2016.04.041.
[34] WANG Xue-bin, LI Shuai-shuai, ADEOSUN A, LI Y, VUJANOVIC M, TAN Hou-zhang, DUIC N. Effect of potassium-doping and oxygen concentration on soot oxidation in O2/CO2 atmosphere: A kinetics study by thermogravimetric analysis [J]. Energy Conversion and Management, 2017, 149: 686-697. DOI:10.1016/j.enconman.2017.01.003.
[35] CHONG H S, AGGARWAL S K, LEE K O, YANG S Y, SEONG H. Experimental investigation on the oxidation characteristics of diesel particulates relevant to DPF regeneration [J]. Combustion Science and Technology, 2013, 185(1): 95-121. DOI: 10.1080/00102202.2012.709563.
[36] YEHLIU K, WAL R V, BOEHMAN A L. Development of an HRTEM image analysis method to quantify carbon nanostructure [J]. Combustion and Flame, 2011, 158(9): 1837-1851. DOI: 10.1016/j.combustflame.2011.01.009.
[37] YEHLIU K, WAL R V, BOEHMAN A L. A comparison of soot nanostructure obtained using two high resolution transmission electron microscopy image analysis algorithms [J]. Carbon, 2011, 49(13): 4256-4268. DOI: 10.1016/j.carbon. 2011.06.003.
[38] WAL R V, TOMASEK A J, STREET K, HULL D R, THOMPSON W K. Carbon nanostructure examined by lattice fringe analysis of high-resolution transmission electron microscopy images [J]. Applied Spectroscopy, 2004, 58(2): 230-237. https://www.osapublishing.org/as/abstract.cfm? uri=as-58-2-230
[39] WAL R V, TOMASEK A J. Soot oxidation [J]. Combustion and Flame, 2003, 134(1, 2): 1-9. DOI: 10.1016/s0010-2180(03)00084-1.
[40] WAL R V, YEZERETS A, CURRIER N W, KIM D H, WANG C M. HRTEM Study of diesel soot collected from diesel particulate filters [J]. Carbon, 2007, 45(1): 70-77. DOI: 10.1016/j.carbon.2006.08.005.
[41] WANG Chang-an, HUDDLE T, HUANG C H, ZHU W, WAL R V, LESTER E H, MATHEWS J P. Improved quantification of curvature in high-resolution transmission electron microscopy lattice fringe micrographs of soots [J]. Carbon, 2017, 117: 174-181. DOI: 10.1016/j.carbon.2017.02.059.
[42] CHENG H K F, CHONG M F, LIU E, ZHOU K, LI L. Thermal decomposition kinetics of multiwalled carbon nanotube/polypropylene nanocomposites [J]. Journal of Thermal Analysis and Calorimetry, 2014, 117(1): 63-71. DOI: 10.1007/s10973-014-3668-8.
[43] KHOLGHY M R, VESHKINI A, THOMSON M J. The core–shell internal nanostructure of soot–A criterion to model soot maturity [J]. Carbon, 2016, 100: 508-536. DOI: 10.1016/ j.carbon.2016.01.022.
[44] TAN Pi-qiang, LI Yuan, SHEN Han-yan. Effect of lubricant sulfur on the morphology and elemental composition of diesel exhaust particles [J]. Jounal of Environmental Sciences, 2017, 55: 354-362. DOI: 10.1016/j.jes.2017.01.014.
[45] KAMEYA Y, HAYASHI T, MOTOSUKE M. Oxidation-resistant graphitic surface nanostructure of carbon black developed by ethanol thermal decomposition [J]. Diamond and Related Materials, 2016, 65: 26-31. DOI: 10.1016/ j.diamond.2016.01.002.
(Edited by FANG Jing-hua)
中文导读
热老化对柴油机干碳烟氧化性能和纳米结构的影响
摘要:柴油机碳烟在高排气温度下会发生热老化,难以通过再生过程去除。本文基于热重(TG)分析和高分辨透射电镜(HRTEM)成像,研究了老化温度、老化时间和氧浓度对碳烟氧化特性的影响。碳烟的活化能随老化温度和氧浓度的增加而增加。当老化时间少于45 min时活化能迅速增加,老化时间处于45~60 min时活化能稳定在157 kJ/mol左右。通过观察碳烟纳米结构可以发现:与未经过热老化的碳烟相比,老化后的碳烟颗粒直径更短,呈更规则的圆形。随着老化温度、老化时间和氧浓度的增加,基本碳粒的“壳-核”结构更稳定。经过热老化后,碳烟边缘长度增加,弯曲度和微晶层间距减小,碳烟纳米结构紊乱程度加深,碳烟的氧化活性降低。因此,优化主动再生策略应避免热老化过程。
关键词:热老化;氧化性能;碳烟纳米结构;活化能
Foundation item: Project(51676167) supported by the National Natural Science Foundation of China; Project(17TD0035) supported by the Sichuan Provincial Scientific Research Innovation Team Program, China; Projects(2017TD0026, 2015TD0021) supported by Science & Technology Department of Sichuan Province, China
Received date: 2019-05-20; Accepted date: 2020-06-08
Corresponding author: FANG Jia, PhD, Associate Professor; Tel: +86-28-87726799; E-mail: jiafang@mail.xhu.edu.cn; ORCID: https://orcid.org/0000-0001-6843-3791

