文章编号:1004-0609(2015)08-2196-09
Ag3PO4/Ni纳米薄膜降解罗丹明B的光电催化性能和反应机理
李爱昌,赵 娣,刘盼盼,孙少敏,刘 萌
(廊坊师范学院 化学与材料科学学院,廊坊 065000)
摘 要:采用电化学方法制备Ag3PO4/Ni薄膜,以扫描电镜(SEM)、X射线衍射(XRD)和紫外-可见漫反射光谱(UV-Vis DRS)对薄膜的表面形貌、晶相结构、光谱特性及能带结构进行表征,以罗丹明B为模拟污染物对薄膜的光电催化活性和稳定性进行测定,采用向溶液中加入活性物种捕获剂和通氮除氧方法对薄膜的光催化降解机理进行探索,并提出光电催化降解罗丹明B的反应机理。结果表明:最佳工艺下制备的Ag3PO4/Ni薄膜具有致密的层状表面结构,是由多晶纳米颗粒构成的薄膜。该薄膜具有显著的光电催化活性,在最佳阳极偏压下,光电催化罗丹明B的降解率是多孔P25 TiO2/ITO薄膜的6.69倍;相对于未加偏压的光催化,降解率提高了5.34倍,并且具有突出的光电协同效应。同时,该薄膜具有优异的光催化和光电催化稳定性。在0.1 V阳极偏压下,可使光催化稳定性提高近一倍。
关键词:Ag3PO4/Ni薄膜;阳极偏压;光电催化;罗丹明B;反应机理
中图分类号:O643;TG 146.3 文献标志码:A
Photoelectrocatalytic property and reaction mechanism of Ag3PO4/Ni nano thin film for degrading rhodamine B
LI Ai-chang, ZHAO Di, LIU Pan-pan, SUN Shao-min, LIU Meng
(Faculty of Chemistry and Material Science, Langfang Teachers College, Langfang 065000, China)
Abstract: Ag3PO4/Ni thin films were prepared by electrochemical method. The surface morphology, phase structure, optical characteristics and band structure of the thin film were analyzed by scanning electron microscopy (SEM), X-ray diffraction (XRD) and ultraviolet-visible diffuse reflectance spectroscopy (UV-Vis DRS), respectively. The photoelectrocatalytic properties and stability of this coating were evaluated with rhodamine B(RhB) as a model compound. Through adding active species scavenger and removing oxygen with nitrogen to the solution, the mechanism of photoelectrocatalytic degradation of the film was explored. The mechanisms of photoeletrocatalytic reaction on the film for RhB under visible irradiation were inputted. The results show that, under optimum conditions, the Ag3PO4/Ni thin film prepared is composed of nano particles and it has dense and layered surface structure. The film has high photoelectrocatalytic activity. At optimum anodic bias, the photoeletrocatalytic degradation rate of Ag3PO4/Ni thin film is 6.69 times as that of porous P25 TiO2 /ITO nanofilm. Compared the coating without anodic bias, the photoeletrocatalytic degradation rate for the Ag3PO4/Ni thin film to RhB increases by 5.34 times and the thin film has obvious photoelectric synergistic effect. Especially, the film has excellent photocatalytic and photoelectrocatalytic stability. At 0.1 V anodic bias, its photoeletrocatalytic stability increases by about one time than its photocatalytic stability without anodic bias.
Key words: Ag3PO4/Ni thin film; anodic bias; photoelectrocatalysis; rhodamine B; reaction mechanism
当前环境问题日趋严重,半导体光催化剂为解决这一问题提供了一种绿色途径, 因而引起了人们广泛的关注[1-3]。与传统技术相比,这种光催化技术具有矿化率高、二次污染少等优势,有望成为下一代环保新技术[4]。 在众多半导体催化剂中,TiO2 由于具有廉价、无毒、化学惰性、稳定性高等[5]特点,使之成为研究最为广泛的光催化剂。然而,TiO2半导体带隙较宽,仅对紫外光有响应,故纯TiO2光催化剂存在光能利用率低的问题。此外量子效率不高这一因素也限制了TiO2 的应用[6]。因此,开发新型高效的宽波段响应型光催化剂成为最近该领域的研究热点。 大量研究致力于对TiO2的修饰改性[7-8],常用的方法为金属及非金属离子掺杂、半导体复合、有机染料光敏化等,从而拓展吸收带边、增强光致电荷的分离和传输,以达到提高催化剂量子效率的目的。 一方面大量研究致力于对TiO2的修饰改性;另一方面,则是探寻、制备具有全新组成的窄带隙光催化材料体系[6]。Ag3PO4是近期发现的一种高效可见光响应的光催化剂。该催化剂对水中析氧和有机染料降解具有极高的催化活性。特别是它的量子效率达到90%以上,远高于大多数光催化剂的。然而,它存在结构不稳定的缺点,主要表现为Ag3PO4微溶于水和在不加入AgNO3电子接收剂的光催化反应中发生光腐蚀。自2010年日本叶金花研究组首次报道该催化剂以来[9],包括本文作者课题组在内的国内外许多学者相继对该材料进行了较深入的研究[10-21]。
当前人们对Ag3PO4的研究还处于粉末催化剂的制备方法及其光稳定性能探究的初步阶段。从制备方法上来说,主要有离子交换法,其次是少量的溶剂热法[22]和银模板法[11],制备方法还比较单一。另外,Ag3PO4负载型催化剂特别是薄膜负载型催化剂的制备及其光催化和光电催化性能研究鲜见报道。 就提高Ag3PO4光稳定性能方面来说,当前科研人员所采用的方法主要有两个:一是在其表面包覆一层溶解度更小的物质[13, 19-20](如卤化银和二氧化锡);二是采用H3PO4和H2O2溶液与使用过的Ag3PO4作用,使光催化过程中产生的金属Ag重新转化为Ag3PO4[15]。事实上,对于Ag3PO4薄膜催化剂而言,完全可以施加一个适当的正偏压来解决光催化稳定性的问题,同时,也有效加速光催化反应。本文作者采用电化学方法在金属Ni上沉积制备高效稳定的Ag3PO4薄膜,以罗丹明B为模拟污染物研究不同偏压对薄膜光电催化活性和稳定性的影响,并探索光电催化机理。
1 实验
1.1 Ag3PO4/Ni薄膜的制备
实验所用主要化学试剂为AgNO3(纯度99.8%,质量分数)、KCl(纯度99.5%,质量分数)、海因(C3H4N2O2,纯度98.0%,质量分数)、(NH4)3PO4·3H2O(纯度98.0%,质量分数),均为分析纯。
首先制备Ag/Ni薄膜[23]。采用上述试剂制备镀液,溶液组成为AgNO3 17g/L、KCl 9 g/L、海因40 g/L。用KOH溶液和稀HNO3调节溶液的pH值为10.5。将80 mL溶液注入自制的恒温镀槽中,镀液的温度由501A型超级数显恒温水浴( 上海浦东荣丰科学仪器公司)控制,以JWL-30Ⅲ型直流稳流器(上海第二电表厂)为电源,采用恒电流沉积。不锈钢板(4 cm×3 cm)作阳极,镍片(1 cm×2 cm)作阴极,阴极非工作面用绝缘胶带封固,两极之间的距离为3 cm,电沉积时间为30 min,其他实验参数为电流密度2.0 mA/cm2;镀液温度25 ℃。
第二步将Ag/Ni薄膜转化为Ag3PO4/Ni薄膜。以上述Ag/Ni薄膜为阳极,不锈钢片为阴极,(NH4)3PO4溶液为电解液进行电解沉积。(NH4)3PO4溶液的浓度为0.8 mol/L、pH值为8.0;电解电流密度为2.5 mA/cm2;沉积时间(以刚形成完整表面膜(此时电流突然下降)为计时零点)为30 s。所制Ag3PO4/Ni薄膜的后处理条件为70 ℃下保温1 h。
1.2 多孔 P25 TiO2/ITO薄膜的制备
参照文献[24],以电泳法在最佳工艺条件下制备多孔P25 TiO2/ITO纳米薄膜(本实验制备薄膜面积为1.0 cm×2.0 cm,其他均与文献[24]相同),以此作参考薄膜来说明Ag3PO4/Ni薄膜的光电催化性能。
1.3 薄膜表征
采用日本理学生产的D/max-RB型X射线衍射仪(XRD)分析薄膜的结构,Cu Kα辐射,掠角为3°。采用德国LEO公司生产的1530型热场发射扫描电镜(SEM)观察薄膜的表面形貌。采用英国Oxford 7426型能谱仪(EDS)测定薄膜表面的组成。采用日本JASCO 公司生产的V-570型紫外-可见-近红外分光光度计测定紫外-可见漫反射光谱(UV-Vis DRS)。
1.4 光催化实验
室温(25 ℃)下,将14 mL浓度为5 mg/L、pH=7的罗丹明B溶液注入硬质试管(7 cm×2 cm)中,将一片尺寸为1.0 cm×2.0 cm的Ag3PO4/Ni薄膜(或同尺寸的TiO2/ITO薄膜)浸入溶液中并使其保持垂直状态。 用150 W卤钨灯为光源,使用滤光片得到最小波长为420 nm的可见光,薄膜与光源距离为18.5 cm,氧气流量为50 mL/min。在通氧和暗态条件下使薄膜吸附20 min,然后开启光源进行光催化实验。每间隔一定时间取样,用722G型分光光度计(波长为552 nm)测定罗丹明B的吸光度,并以此计算罗丹明B的降解率。
1.5 光电催化实验
以Ag3PO4/Ni薄膜电极为工作电极,Pt片为辅助电极,饱和甘汞电极(Saturated calomel electrode,SCE)为参比电极构成三电极系统。在上述罗丹明B溶液中加入Na2SO4支持电解质,使Na2SO4浓度为0.5 mol/L。外加偏压通过HDV-7型恒电位仪施加。光电催化使用的可见光及其他条件除特别声明外与光催化实验 相同。
2 结果与讨论
2.1 薄膜的SEM和EDS表征
图1(a)所示为Ag3PO4/Ni薄膜的SEM像。由图1(a)可见,薄膜结构致密,晶体呈柱状侧向生长,具有层状结构。薄膜表面存在直径约为1~2 μm的孔洞,这是将银膜电解为Ag3PO4薄膜过程中析氧副反应引起的。图1(b)所示为图1(a)更高倍数(50000倍)的SEM像。由图1(b)可见,薄膜表面细腻,未见构成薄膜的更小结构微粒。然而,由图1(b)中标尺可以估测构成薄膜的微粒直径应小于50 nm。这进一步说明制备的薄膜是由纳米颗粒构成且结构致密的薄膜。图1(c)所示为所制薄膜的EDS谱。由图1(c)可见,薄膜含有Ag、P、O与Ni 4种元素。其中,Ag、P与O元素的摩尔分数分别为35.33%、10.62%和54.05%,这与Ag3PO4薄膜Ag、P、O元素的理论值37.5%、12.5%和50%(摩尔分数)接近,说明制备薄膜为Ag3PO4膜。
2.2 薄膜的XRD分析
图2所示为Ag3PO4/Ni薄膜的XRD谱。由图2可知,薄膜的衍射峰位(2θ)依次为20.86°、29.57°、33.34°、36.78°、48.01°、52.89°、57.39°、77.78°,由此判定所制备的Ag3PO4晶体为体心立方晶系(PDF 06-0505)。图2中还出现Ag的衍射峰(PDF 04-0783),由薄膜制备方法可知,这是Ag3PO4薄膜下面有未被氧化的单质银所致。依最强衍射峰按Scherrer公式[25]计算薄膜的平均晶粒尺寸为13.1 nm。
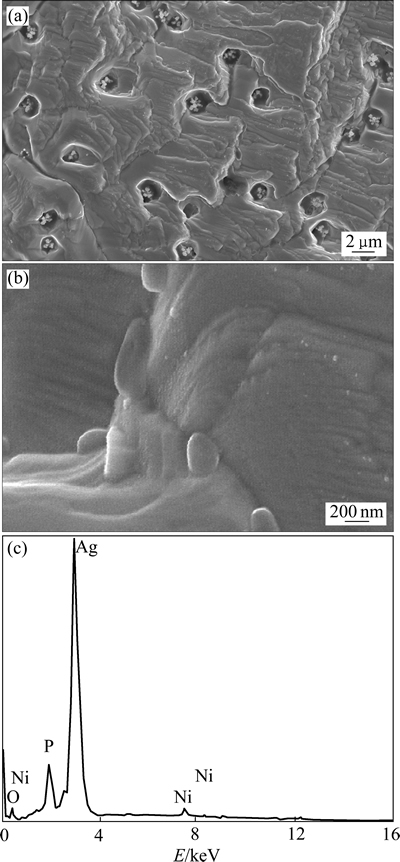
图1 Ag3PO4/Ni薄膜的SEM像和EDS谱
Fig. 1 SEM images((a), (b)) and EDS spectrum of Ag3PO4/Ni thin film(c)
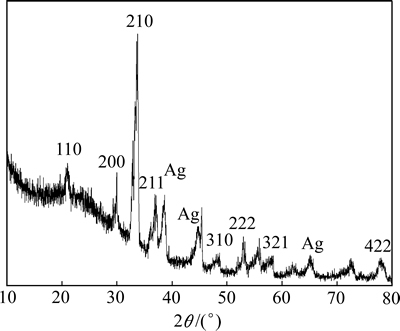
图2 Ag3PO4/Ni薄膜的XRD谱
Fig. 2 XRD spectrum of Ag3PO4/Ni thin
2.3 薄膜的紫外-可见漫反射光谱和能带结构
图3(a)所示为Ag3PO4/Ni薄膜的紫外-可见漫反射光谱(UV-Vis DRS)。由图3(a)可知,薄膜对可见光有明显的吸收。Ag3PO4为间接跃迁半导体[26],带隙能可由DRS数据以(αhν)0.5对(hν)作图获得(见图3(b))。这里的α和ν分别为半导体的吸收系数和被吸收光波的频率。由图3(b)可知,所制薄膜的带隙能为2.29 eV。 半导体的导带底和价带顶能级位置可用经验公 式[27]计算确定,如式(1)和(2)所示:
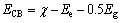 (1)
(1)
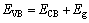 (2)
(2)
式中:ECB和EVB分别为半导体导带底和价带顶所对应的能量(相对于标准氢电极(NHE));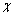 为半导体的电负性,是构成该半导体的各种原子电负性的几何平均值;Eg为半导体的带隙能;Ee为电子在标准氢电极(NHE)的最高占有能级(Fimi能级)的能量[27],其值为4.5 eV(vs Vacuum)。对于所制备的Ag3PO4薄膜,
为半导体的电负性,是构成该半导体的各种原子电负性的几何平均值;Eg为半导体的带隙能;Ee为电子在标准氢电极(NHE)的最高占有能级(Fimi能级)的能量[27],其值为4.5 eV(vs Vacuum)。对于所制备的Ag3PO4薄膜,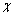 为5.96 eV[28],Eg为2.29 eV,分别代入式(1)和(2)中得到ECB=0.32 eV,EVB=2.61 eV。
为5.96 eV[28],Eg为2.29 eV,分别代入式(1)和(2)中得到ECB=0.32 eV,EVB=2.61 eV。
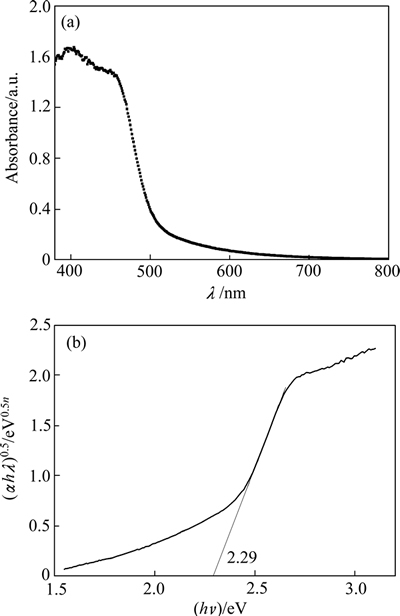
图3 Ag3PO4/Ni 薄膜紫外-可见漫反射光谱和带隙
Fig. 3 UV-Vis diffuse reflectance spectrum(a) and band gap(b) of Ag3PO4/Ni thin film
2.4 外加偏压对薄膜光电催化性能的影响
图4所示为外加阳极偏压对Ag3PO4/Ni薄膜光电催化活性的影响。由图4可以看出,阳极偏压在0~0.3 V范围时,随着偏压的增大,薄膜催化活性不断增大;在0.3 V以后,薄膜催化活性又随着偏压的增大依次减小。Ag3PO4/Ni薄膜在偏压为0.3 V时,表现出最高的光电催化活性,光电催化12 min后,罗丹明B的降解率为86.3%,而未加偏压的光催化降解率只有13.6%,光电催化活性相对于未加偏压的光催化活性提高了5.34倍。此外,由图4还可以看到,在实验所施加的所有偏压范围内(0.1~0.5 V),薄膜的光电催化活性均远大于它的光催化活性。
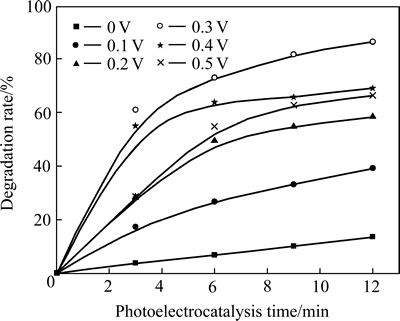
图4 可见光照射下不同外加偏压对Ag3PO4/Ni薄膜光电催化性能的影响
Fig. 4 Effect of different anodic biases on photo- electrocatalytic activity of Ag3PO4/Ni thin film under visible light irradiation
2.5 Ag3PO4薄膜的光电协同效应
光催化、电催化和光电催化都是有催化剂存在才能发生的化学反应。光催化是在光能量场作用下发生的催化反应,光催化剂处于电子激发态。依据反应物的性质和入射光波频率的不同,反应物也可能处于激发态或基态。电催化是在电场作用下发生的催化反应,电催化剂和反应物均处于电子基态。两者的主要区别在于能量作用场和催化剂所处的电子状态不同。光电催化则是在光能量场和电场共同作用下发生的催化反应,其中所用的光电催化剂可能既是光催化剂,也是电催化剂,也可能只是光催化剂而电催化作用不大或没有电催化作用。光电催化剂处于电子激发态。优良的光电催化剂应具有突出的光电协同效应,它是电催化与光催化能动作用的结果。
图5所示为Ag3PO4/Ni薄膜电极的光催化、电催化和光电催化降解罗丹明B性能的比较。光催化或光电催化实验条件仍如上所述。电催化除不开启光源外,其他与光电催化相同。电催化与光电催化所施加的阳极偏压均为0.3 V。
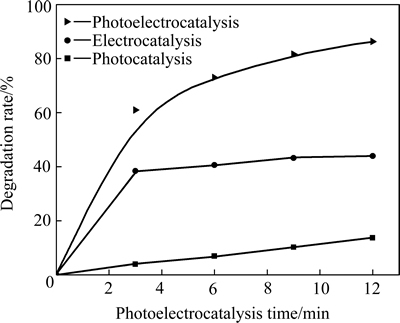
图5 Ag3PO4/Ni薄膜的光催化、电催化和光电催化性能
Fig. 5 Photocatalytic, electrocatalytic and photoelectrocatalytic activities of Ag3PO4/Ni thin film (Applied anode bias is 0.3 V)
由图5可见,在整个实验过程中,Ag3PO4/Ni薄膜电极对罗丹明B的催化降解展现出显著的光电协同效应。催化降解12 min后,单纯光催化使罗丹明B降解了13.6%,电催化的降解了43.9%,光电催化的降解了86.3%,光电催化的降解率是光催化和电催化降解率之和的1.50倍。由图5还可看到,电催化降解作用只发生在开始实验的一个较短时段内(电解曲线斜率可表示反应速率),之后的电催化反应速率几乎为0。换言之,在0.3 V偏压下,Ag3PO4/Ni薄膜电极对于浓度小于3 mg/L或小于4 mg/L(由罗丹明B溶液初始浓度(5 mg/L)和电解曲线水平段的起点降解率估算)的罗丹明B溶液没有电催化降解作用。这表明就整体光电催化反应过程而言,施加的电场只是起到了助光催化的作用。这是Ag3PO4/Ni薄膜电极具有光电协同效应的本质所在。
2.6 Ag3PO4/Ni薄膜与多孔P25 TiO2/ITO薄膜光电催化性能的比较
鉴于TiO2在可见光作用下对染料有一定的光催化降解能力,许多研究者[17,20,22]将可见光催化剂对染料的降解活性与P25 TiO2进行比较。为了说明Ag3PO4/Ni薄膜的光电催化活性,本研究将所制薄膜与P25 TiO2/ITO薄膜进行了对比实验。
图6所示为两薄膜在各自最佳阳极偏压下(Ag3PO4/Ni和P25 TiO2/ITO的最佳偏压分别为0.3 V和0.2V[29])光电催化降解罗丹明B的降解率随催化时间的变化。由图6可见,Ag3PO4/Ni薄膜的光电催化活性远大于TiO2/ITO薄膜的。光电催化3 min后,Ag3PO4/Ni薄膜的降解率(61.0%)是TiO2/ITO薄膜的16.3倍;光电催化作用12 min后,Ag3PO4/Ni薄膜对罗丹明B的降解率高达86.3%,它与TiO2/ITO薄膜的降解率之比仍能达到6.69倍。
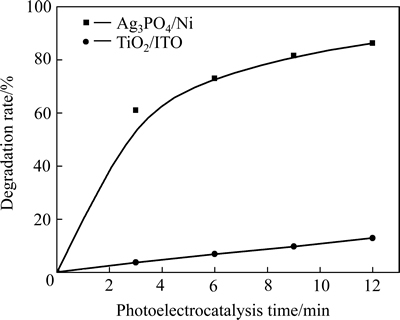
图6 不同薄膜电极对罗丹明B光电催化降解率的影响
Fig. 6 Effect of different thin film electrodes on photoelectrocatalytic degradation rate of rhodamine B
2.7 外加阳极偏压对Ag3PO4/Ni薄膜光催化稳定性的影响
本文作者对施加阳极偏压对光催化稳定性的影响进行了探究。为了避免多次通、断电对Ag3PO4/Ni薄膜光催化稳定性的影响而不掩盖问题的实质,实验采用在大量罗丹明B溶液(140 mL)中进行光催化降解的方法,即光催化稳定性实验所用罗丹明B溶液的体积是前述光催化和光电催化活性实验所用体积的10倍。罗丹明B溶液的初始浓度仍为5 mg/L。其他光催化和光电催化条件与1.4节或1.5节中的相同。此外,由于这种考察薄膜光催化稳定性的方法在大量溶液中进行,光催化反应过程中的浓度效应相对较小,故从曲线斜率的大小就可以判断薄膜在此刻的催化活性或是否失效。
图7所示为外加偏压为0.1 V和不加偏压两种条件下光催化罗丹明B的降解率与催化时间的关系曲线。由图7可见,不加偏压的光催化反应进行到5 h后变为水平线(曲线斜率为0),说明薄膜催化作用失效。而施加0.1 V偏压的光电催化反应进行到10 h后,曲线才趋向水平。这说明对薄膜施加0.1V偏压可使光催化稳定性提高近1倍。外加阳极偏压对Ag3PO4/Ni薄膜的光催化稳定性的提高有显著作用。

图7 不同阳极偏压对Ag3PO4/Ni薄膜光催化稳定性的影响
Fig. 7 Effect of different anodic biases on photocatalytic stability of Ag3PO4/Ni thin film under visible light irradiation (Applied anode bias is 0.1 V. Volume, concentration and pH value of rhodamine B are 140 mL, 5 mg/L and 7, respectively)
实验还表明,过大的阳极偏压会使薄膜与基底在相对较短的时间内分离脱落(如在0.3 V偏压下催化作用30 min后薄膜脱落),这可能是阳极偏压较大时在Ag3PO4/Ni薄膜电极上发生较剧烈的析氧反应引起的。
施加较小的阳极偏压能够提高薄膜光催化稳定性的原理容易理解。众所周知,Ag3PO4光稳定不高的原因是其见光后晶体中的Ag+离子易分解为单质Ag,即发生反应如式(3)所示:
Ag++e→Ag (3)
施加阳极偏压意味着提高了Ag3PO4薄膜的电极电势,因而对上述还原反应有抑制作用。
2.8 Ag3PO4/Ni薄膜光电催化降解机理
为了探索Ag3PO4/Ni薄膜光电催化降解罗丹明B的反应机理,在罗丹明B溶液中加入不同活性氧化物捕获剂,以探测反应系统中生成的活性物种及其对光催化反应的作用。实验中以AgNO3为电子(e)捕获剂,EDTA为空穴(h+)捕获剂,异丙醇作为羟基自由基(·OH)捕获剂,各种捕获剂在反应系统中的初始浓度与模拟污染物罗丹明B的初始浓度之比为100:1,即捕获剂的浓度为1.04×10-3 mol/L。为了考察O2对光电催化反应的影响,也做了通氮除氧实验。实验中施加的阳极偏压为0.3 V,其他光电催化条件与1.5节中的相同。
图8所示为加入不同捕获剂对Ag3PO4/Ni薄膜光电催化降解活性的影响。由图8可见,向溶液中加入h+捕获剂、·OH捕获剂以及向溶液中通入氮除氧都会使罗丹明B的降解率大幅度下降,并且它们下降的幅度相近。与不加任何捕获剂的光电催化系统(曲线a)相比,光电催化9 min后,降解率由81.6%降低至23.0%~27.3%,说明h+、·OH和·O2-是该光电催化反应系统的主要活性氧化物种。由图8还看到,向溶液中加入电子捕获剂(曲线b),罗丹明B的降解率相对于不加任何捕获剂的系统(曲线a)有一定程度的下降。这一实验结果可由图4进行解释。由图4可见,光电催化的最佳阳极偏压为0.3 V,大于此偏压会使光电催化活性降低。由于捕获剂对薄膜光电催化降解活性的影响实验是在最佳偏压下进行的,在恒定电极偏压为0.3 V时加入电子捕获剂所起的作用(见图8)与不加电子捕获剂条件下由偏压为0.3 V继续增加阳极偏压(见图4)的作用是相同的,因而,会出现图8中加入电子捕获剂后降解率下降的结果。
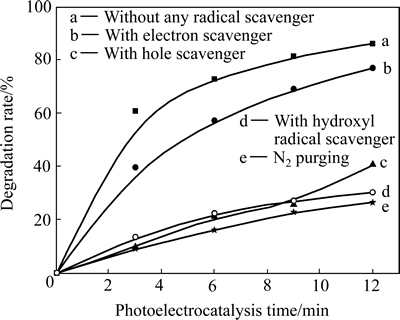
图8 不同捕获剂对Ag3PO4/Ni薄膜光电催化活性的影响
Fig. 8 Effect of different scavengers on photoelectrocatalytic activity of Ag3PO4/Ni thin film under visible light irradiation (Applied anode bias is 0.3 V)
根据文献[30-31]可知,半导体电极电势(或外加偏压)的变化会改变光催化降解反应的机制。粉末状Ag3PO4光催化降解罗丹明B的机理已有报道[14],其主要的活性氧化物种为h+,而不是·OH和·O2-。对于Ag3PO4施加阳极偏压的光催化反应,本研究测定的结果是h+、·OH和·O2-均为主要活性物种。反应机理改变的根本原因是电极电势的变动使得光催化剂表面附近活性物种的种类、被降解物的存在形式和它们的浓度发生了变化。
为了深入研究Ag3PO4/Ni薄膜电极光电催化降解罗丹明B的反应机理,本文作者也对薄膜光催化罗丹明B系统中生成的活性物种进行了测试。为了效果明显,罗丹明B溶液的浓度改为1 mg/L。各种捕获剂在反应系统中的初始浓度与模拟污染物罗丹明B的初始浓度之比仍为100:1,即捕获剂的浓度为2.09×10-4 mol/L。其他光催化条件与1.4节的相同。
测试结果如图9所示。由图9可以看出,h+是光催化反应的主要活性物种(曲线e),·OH不是活性物种(曲线c)。·O2-是否为主要活性物种需要对曲线a、b和d进行分析。图9中曲线a是向溶液中加入电子捕获剂的实验结果,由此可知,加入电子捕获剂后,薄膜催化活性急剧增加,催化作用15 min后,罗丹明B的降解率高达69.3%,是不加任何捕获剂的2.31倍(曲线b),这说明引发光催化反应的主要是阳极活性物种(在价带生成的活性物种),而不是阴极活性物种(在导带产生的活性物种)。图9中曲线d是向溶液中通氮除氧的实验结果,可见罗丹明B的降解率(曲线d)相对于不加捕获剂的系统(曲线b)显著降低,但是降低的程度明显不及加入h+捕获剂的系统(曲线e)。这一方面说明溶解氧是薄膜光催化反应的电子捕获剂,对薄膜光生电荷的分离有突出的促进作用;另一方面表明由此生成的超氧自由基(·O2-)对光催化降解罗丹明B的活性较小。简言之,Ag3PO4/Ni薄膜光催化降解罗丹明B 的主要活性物种是h+,而不是·OH和·O2-。这与文献[14]的结论一致,但也有不同。GE等[14]认为Ag3PO4光催化系统中不可能生成·OH和·O2-,并对其热力学不可能性进行了相关计算。本文作者认为这种计算是不准确的,在反应系统中不是不能生成·OH和·O2-,而是它们的降解活性很小或基本没有降解作用。文献[14]的问题主要表现在使用标准电极电势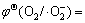 0.13 V或
0.13 V或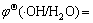 2.68 V取代相应的电极电势并对Ag3PO4的导带底或价带顶势进行比较上面。以下仅以超氧自由基(·O2-)为例进行说明。
2.68 V取代相应的电极电势并对Ag3PO4的导带底或价带顶势进行比较上面。以下仅以超氧自由基(·O2-)为例进行说明。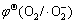 是标准电极电势,是指O2的压力为标准压力,·O2-的活度为1时的电极电势。在光催化反应系统中,由于·O2-为活性自由基,实际存在的浓度通常小于10-7~10-8 mol/L,把此值作为·O2-的活度代入Nernst公式[32]中进行计算可得
是标准电极电势,是指O2的压力为标准压力,·O2-的活度为1时的电极电势。在光催化反应系统中,由于·O2-为活性自由基,实际存在的浓度通常小于10-7~10-8 mol/L,把此值作为·O2-的活度代入Nernst公式[32]中进行计算可得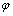 (O2/·O2-)为0.54~0.60 V,此值远大于Ag3PO4的导带底势(或者说电对O2/·O2-的费米能级远低于Ag3PO4导带底的能级位置;本实验所制Ag3PO4薄膜的导带底势为0.32 V),故从热力学角度可以生成·O2-。事实上,本研究已证实不仅在热力学上是可行的,动力学上也是能够实现的。同理,按上述计算方法可得
(O2/·O2-)为0.54~0.60 V,此值远大于Ag3PO4的导带底势(或者说电对O2/·O2-的费米能级远低于Ag3PO4导带底的能级位置;本实验所制Ag3PO4薄膜的导带底势为0.32 V),故从热力学角度可以生成·O2-。事实上,本研究已证实不仅在热力学上是可行的,动力学上也是能够实现的。同理,按上述计算方法可得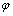 (·OH/H2O)为2.21~2.27 V(计算中未考虑实际溶液pH值影响,若考虑溶液pH值接近中性,则此电极的电极电势更小),此值远小于本文作者所制Ag3PO4薄膜的价带顶势(2.61 V),故从热力学上也可以生成·OH。
(·OH/H2O)为2.21~2.27 V(计算中未考虑实际溶液pH值影响,若考虑溶液pH值接近中性,则此电极的电极电势更小),此值远小于本文作者所制Ag3PO4薄膜的价带顶势(2.61 V),故从热力学上也可以生成·OH。
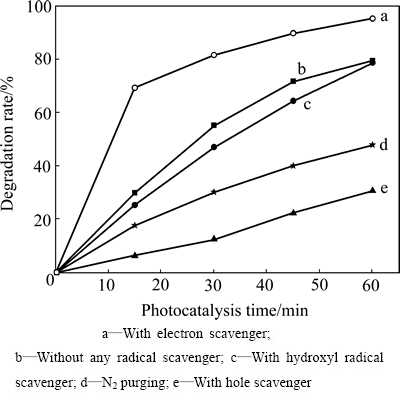
图9 不同捕获剂对Ag3PO4/Ni薄膜光催化活性的影响
Fig. 9 Effect of different scavengers on photocatalytic activity of Ag3PO4/Ni thin film
·O2-和·OH对于罗丹明B没有明显的降解活性与罗丹明B(RhB)在催化剂表面附近的存在形式有关。已有的研究成果[30, 33]表明,式(4)为快反应,式(5)为较慢的反应:
·O2-/·OH+RhB+·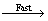 M (4)
M (4)
·O2-/·OH+RhB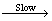 M (5)
M (5)
式中:M为反应的降解产物。
在可见光照射下,Ag3PO4催化剂中的电子自价带跃迁至导带,导带中电子浓度的增大不利于罗丹明B正离子自由基RhB+·的生成(RhB+ →RhB*; RhB*→RhB+·+e),因而,催化剂表面附近RhB+·浓度很小,进而导致·O2-和·OH对罗丹明B的活性极低。
→RhB*; RhB*→RhB+·+e),因而,催化剂表面附近RhB+·浓度很小,进而导致·O2-和·OH对罗丹明B的活性极低。
对于Ag3PO4/Ni薄膜施加阳极偏压的光电催化系统,电极电势的升高使得电极表面附近RhB+·浓度增大,从而使式(4)的速率加快,故表现为·O2-和·OH对罗丹明B有较强的降解活性。
综合上述分析,提出可见光作用下Ag3PO4/Ni薄膜光电催化降解罗丹明B的反应机理为
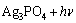 →
→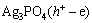 (6)
(6)
光阴极反应:
RhB+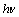 →RhB* (7)
→RhB* (7)
RhB*+Ag3PO4→Ag3PO4(e)+RhB+· (8)
Ag3PO4(e)+O2→ ·O2-+Ag3PO4 (9)
Ag3PO4(e)→Ag3PO4+e (10)
·O2-+RhB+·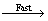 M (11)
M (11)
·O2-+RhB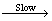 M (12)
M (12)
光阳极反应:
Ag3PO4(h+)+RhB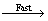 MF+Ag3PO4 (13)
MF+Ag3PO4 (13)
Ag3PO4(h+)+H2O→H++·OH+Ag3PO4 (14)
·OH+ RhB+·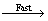 M (15)
M (15)
·OH+ RhB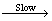 M (16)
M (16)
式中:RhB*表示罗丹明B的电子激发态;RhB+·表示罗丹明B自由基。式(6)和(7)分别表示Ag3PO4和罗丹明B吸收可见光被激发;式(10)表示Ag3PO4导带中的部分电子在阳极偏压作用下迁移至外电路。
另外,由上述机理容易解释图4所示偏压对光催化活性影响的规律。显然,阳极偏压下光催化活性的提高主要是由RhB+·浓度的增大引起的,此时3种活性物种(h+、·OH和·O2-)均有较强的降解活性;而过高的阳极偏压会使·O2-物种的浓度大大减小甚至消失(由式(9)的速率显著降低引起),此时在价带由式(14)产生的过高浓度的·OH也会因会互碰作用而使其浓度降 低[34],从而造成光催化降解率相对下降。
3 结论
1) 采用电化学方法制备了Ag3PO4/Ni纳米薄膜,该薄膜具有致密的层状表面结构,薄膜具有显著的光电催化活性。在最佳阳极偏压下,光电催化罗丹明B的降解率是多孔P25 TiO2/ITO薄膜的6.69倍;相对于未加偏压的光催化,降解率提高了5.34倍,并且具有突出的光电协同效应。
2) 薄膜具有良好的光催化稳定性和优异的光电催化稳定性。在0.1 V阳极偏压下,可使光催化稳定性提高近一倍。
3) 较详细探讨薄膜光电催化降解罗丹明B的反应机理。此种同时提高Ag3PO4/Ni薄膜光催化活性和稳定性的方法以及有关机理,原则上也适用于其他银基半导体光催化剂。
REFERENCES
[1] Antoniadou M, Lianos P. Photoelectrochemical oxidation of organic substances over nanocrystalline titania: Optimization of the photoelectrochemical cell[J]. Catalysis Today, 2009, 144: 166-171.
[2] Szabo-Bardos E, Zsilak Z, Horvath O. Photocatalytic degradation of anionic surfactant in titanium dioxide suspension[J]. Prog Colloid Polym Sci, 2008, 135: 21-28.
[3] María-José L M, Rafael v G, José A. Role of the support on the activity of silica-supported TiO2 photocatalysts: Structure of the TiO2/SBA-15 photocatalysts[J]. Catalysis Today, 2005, 101: 307-314.
[4] 许宜铭. 环境污染物的光催化降解: 活性物种与反应机理[J].化学进展, 2009, 21(2/3): 524-533.
XU Yi-ming. Photocatalytic degradation of environmental pollutants: The active species and reaction mechanism[J]. Progress of Chemistry, 2009, 21(2/3): 524-533.
[5] Addamo M, Augugliaro V, García-López E, LODDO V, Marcì G, Palmisano L. Oxidation of oxalate ion in aqueous of TiO2 by photocatalysis and ozonation[J]. Catalysis Today, 2005, 107 /108: 612-618.
[6] 金 超, 秦 瑶, 杨金虎. 新型非TiO2半导体光催化剂[J]. 化学进展, 2014, 26(2/3): 225-233.
JIN Chao, QIN Yao, YANG Jin-hu. Novel non-TiO2 semiconductor photocatalysts[J]. Progress of Chemistry, 2014, 26(2/3): 225-233.
[7] Zou Z, YE J, Sayama K, Arakawa H. Direct splitting of water under visible light irradiation with an oxide emiconductor photocatalyst[J]. Nature, 200l, 414(6864): 625-627.
[8] 鲁 飞, 盂凡明. TiO2光催化剂掺杂改性研究进展[J]. 硅酸盐通报, 2011, 30(1): 116-119.
LU Fei, Meng Fan-ming. Research evolution of doping modification on TiO2 photocatalyst[J]. Bulletin of the Chinese Ceramic Society, 2011, 30(1): 116-119.
[9] YI Z G, YE J H, Kikugawa N, Kikugawa N, Kako T, Ouyang S X, Stuary-Williams H, Yang H, Cao J Y, Luo W J, Li Z S, Liu Y, Withers R L. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation[J]. Nat Mater, 2010, 9(7): 559-564.
[10] BI Y P, HU H Y, OUYANG S X, LU G X, CAO J Y, YE J H. Photocatalytic and photoelectric properties of cubic Ag3PO4 sub-microcrystals with sharp corners and edges[J]. Chem Comm, 2012, 48(31): 3748-3750.
[11] Bi Y P, Hu H Y, Ouyang S X, Jiao Z B, Lu G X, Ye J H. Selective growth of Ag3PO4 submicro-cubes on Ag nanowires to fabricate necklace-like heterostructures for photocatalytic applications[J]. Journal of Materials Chemistry, 2012, 22(30): 14847-14850.
[12] Bi Y P, Ouyang S X, Umezawa N, Cao J Y, Ye J H. Facet effect of single crystalline Ag3PO4 sub-microcrystals on photocatalytic properties[J]. Journal of the American Chemical Society, 2011, 133(17): 6490-6492.
[13] Bi Y P, Ouyang S X, Ye J H. Facile synthesis of rhombic dodecahedral AgX/Ag3PO4(X=Cl, Br, I)heterocrystals with enhanced photocatalytic properties and stabilities[J]. Physical Chemistry Chemical Physics, 2011, 13(21): 10071-10075.
[14] Ge M, Zhu N, Zhao Y P, Li J, Liu L. Sunlight-assisted degradation of dye pollutants in Ag3PO4 suspension[J]. Ind Eng Chem Res, 2012, 51(14): 5167-5173.
[15] Wang H, Bai Y S, Yang J T, Lang X F, Li J H, Guo L. A facile way to rejuvenate Ag3PO4 as a recyclable highly efficient photocatalyst[J]. Chemistry: A European Journal, 2012, 18(18): 5524-5529.
[16] Wang W G, Cheng B, Yu J G, Liu G, Fan W H. Visible-light photocatalytic activity and deactivation mechanism of Ag3PO4 spherical particles[J]. Chemistry: An Asian Journal, 2012, 7(8): 1902-1908.
[17] Liang Q H, Ma W J, Yang X M, Li Z, Yang X M. Hierarchical Ag3PO4 porous microcubes with enhanced photocatalytic properties synthesized with the assistance of trisodium citrate[J]. Cryst Eng Comm, 2012, 14(8): 2966-2973.
[18] Khan A, Qamar M, Muneer M. Synthesis of highly active visible-light-driven colloidal silver orthophosphate[J]. Chemical Physics Letters, 2012, 20(25): 54-58.
[19] Cao J, Luo B D, Lin H L, Chen S F. Visible light photocatalytic activity enhancement and mechanism of AgBr/Ag3PO4 hybrids for degradation of methylorange[J]. Journal of Hazardous Materials, 2012, 4(1): 107-115.
[20] Zhang L L, Zhang H C, Huang H, Liu Y, Kang Z H. Ag3PO4/SnO2 semiconductor nanocomposites with enhanced photocatalytic activity and stability[J]. New Journal of Chemistry, 2012, 36(8): 1541-1544.
[21] MA Y Z, CHENG F, LIU W S, WANG J, WANG Y K. Research progress of Ag3PO4-based photocatalyst: Fundamentals and performance enhancement[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(1): 112-121.
[22] Dinh C T, Nguyen T D, Kleitz F, Do T O. Large-scale synthesis of uniform silver orthophosphate colloidal nanocrystals exhibiting high visible light photocatalytic activity[J]. Chemistry Communication, 2011, 47(32): 7797-7799.
[23] 杨勇彪, 张正富, 陈庆华, 徐明丽, 马全宝. 铜基无氰镀银的研究[J]. 云南冶金, 2004, 33(4): 20-22, 31.
Yang Yong-biao, Zhang Zheng-fu F, Chan Qing-hua, Xu Ming-li, Ma Quan-bao. The study on cyanide-free silver electroplating on copper substrate[J]. Yunnan Metallurgy, 2004, 33(4): 20-22,31.
[24] 李爱昌, 卢艳红, 陈荣英, 路丽丽, 郑彦俊.电泳法制备TiO2/MWCNTs纳米薄膜及其光催化罗丹明B的性能[J]. 硅酸盐学报, 2014, 42(6): 808-815.
Li Ai-chang, Lu Yan-hong, Chen Rong-ying, Lu Li-li, Zheng Yan-jun. Preparation of TiO2/MWCNTs nanocomposite films by electrophoretic deposition and their photocatalytic activity for Rhodamine B[J]. Journal of Chinese Ceramic Society, 2014, 42(6): 808-815.
[25] 范 雄. 金属X射线衍射学[M]. 北京: 机械工业出版社, 1996: 45.
FAN Xiong. Metal X-ray diffractometry[M]. Beijing: Mechanical Industry Press, 1996: 45.
[26] Liu J J, Fu X L, Chen S F, Zhu Y F. Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method[J]. Applied Physics Letters, 2011, 99: 191903-1-3.
[27] 吴浩青, 李永舫. 电化学动力学[M]. 北京: 高等教育出版社, 1998: 178.
WU Hao-qing, LI Yong-fang. Electrochemical kinetics[M]. Beijing: China Higher Education Press, 1998: 178.
[28] 刘建新, 王韵芳, 王雅文, 樊彩梅. Ag/Ag3PO4/g-C3N4复合光催化剂的合成与再生及其可见光下的光催化性能[J]. 物理化学学报, 2014, 30(4): 729-737.
Liu Jian-xin, Wang Yun-fang, Wang Ya-wen, Fan Cai-mei. Synthesis, regeneration and photocatalytic activity under visible-light irradiation of Ag/Ag3PO4/g-C3N4 hybrid photocatalysts[J]. Acta Phys-Chim Sin, 2014, 30(4): 729-737.
[29] 李爱昌, 卢艳红, 曹国蕾, 刘 萍, 刘增仁. (Ni-Mo)/TiO2纳米薄膜光电催化降解罗丹明B的性能[J]. 硅酸盐学报, 2011, 39(8): 1361-1367.
LI Ai-chang, LU Yan-hong, CAO Guo-lei, LIU Ping, LIU Zeng-ren. Photoelectrocatalytic activity for Rhodamine B of (Ni-Mo)-TiO2 thin film[J]. Journal of Chinese Ceramic Society, 2011, 39(8): 1361-1367.
[30] 杨 娟, 戴 俊, 赵进才, 缪 娟. 负偏压作用下TiO2膜电极光催化降解染料MG的机理研究[J]. 科学通报, 2009, 54(15): 2196-2204.
YANG Juan, DAI Jun, ZHAO Jin-cai, MIU Juan. Mechanism of photocatalytic degradation of dye MG by TiO2 -film electrode with cathodic bias potential[J]. Chinese Science Bulletin, 2009, 54(15): 2196-2204.
[31] 李爱昌, 李健飞, 刘亚录, 张建平, 赵丽平, 卢艳红. 负偏压下(Ni-Mo)/TiO2膜电极光电催化降解罗丹明B的性能和机理[J]. 化学学报, 2013, 71(5): 815-821.
LI Ai-chang, LI Jian-fei, LIU Ya-lu, ZHANG Jian-ping, ZHAO Li-ping, LU Yan-hong. Photoelectrocatalytic property and reaction Mechanism of (Ni-Mo)/TiO2 nano thin film at negative bias for Rhodamine B[J]. Acta Chimica Cinica, 2013, 71(5): 815-821.
[32] 郭鹤桐, 刘淑兰. 理论电化学[M]. 北京: 宇航出版社, 1984.
GUO He-tong, LIU Shu-lan. Theoretical electrochemistry[M]. Beijing: China Aerospace Press, 1984.
[33] 杨 娟, 缪 娟, 戴 俊, 李建通. 可见光照射下纳晶TiO2电极对染料污染物的光电催化降解研究[J]. 河南理工大学学报, 2010, 29(2): 259-265.
YANG Juan, DAI Jun, MIU Juan, LI Jian-tong. Photoelectrocatalytic degradation of dye pollutants with nano-TiO2 electrode under visible-light irradiation[J]. Journal of Henan Polytechnic University, 2010, 29(2): 259-265.
[34] Sun C C, Chou T C. Kinetics and mechanism of photoelectrochemical oxidation of nitrite ion by using the rutile from of a TiO2/Ti photoelectrode with high electric field enhancement[J]. Ind Eng Chem Res, 1998, 37(11): 4027-4214.
(编辑 李艳红)
基金项目:河北省科技支撑计划项目(11276732);廊坊师范学院重点科学研究项目(LSZZ201303)
收稿日期:2015-01-06;修订日期:2015-05-20
通信作者:李爱昌,教授;电话:0316-2188370;E-mail: aichangli@hotmail.com