Hg2+对木瓜蛋白酶的修饰作用及其动力学
蔡西玲,曾虹燕,蔡联辉,何平,张存滢,刘学英,吴雅兰
(湘潭大学 化工学院,湖南 湘潭,411105)
摘要:通过对Hg2+处理的木瓜蛋白酶FT-IR图谱中的酰胺Ⅰ带进行去卷积和曲线拟合,结合荧光光谱技术,对其进行二级结构分析。运用邹氏酶活性不可逆改变动力学理论,研究酶与Hg2+结合的动力学规律,探索Hg2+对木瓜蛋白酶活性的作用机理。研究结果表明:Hg2+对木瓜蛋白酶具激活和抑制的双重作用,Hg2+对木瓜蛋白酶作用表现出低促高抑的Hormesis现象。酶活主要取决于其活性中心位构象,尤其是无规则卷曲和β-转角。β-转角含量减少,无规则卷曲含量增加,有助于酶活提高,反之酶活降低。低浓度下(10-6 mol/L)Hg2+对木瓜蛋白酶的作用为非竞争性不可逆激活,酶活增大;高浓度下(10-4 mol/L) Hg2+对酶的抑制作用以竞争性抑制为主,酶活降低。
关键词:木瓜蛋白酶;Hg2+;动力学;酶活;二级结构;不可逆修饰
中图分类号:TQ028.8 文献标志码:A 文章编号:1672-7207(2013)10-3991-07
Modification effect of Hg2+ on papain and its kinetics
CAI Xiling, ZENG Hongyan, CAI Lianhui, HE Ping, ZHANG Cunying, LIU Xueying, WU Yalan
(School of Chemical Engineering, Xiangtan University, Xiangtan 411105, China)
Abstract: The secondary structures of the papain treated by Hg2+ were determined by FT-IR in amide-I region band using Fourier deconvolution and curve-fitting technique and fluorescence emission spectra. Based on Tsou’s theory on the kinetics of irreversible modification of enzymic activity, the kinetics of the reaction of Hg2+ with papain was studied in order to explore the mechanism of Hg2+ on papain. The results show that Hg2+ has active and inhibitory effect on papain, and the effect of Hg2+ on papain in the tyrosine hydrolytic reaction shows the Hormesis effect. The activity of papain depends crucially on the active site conformation, especially of β-turn and random. The papain activity is increased when the amounts of β-turn decrease or with random increase in papain, and vice versa. At low Hg2+ concentration of 10-6 mol/L, Hg2+ is efficacious activator, and effect is classified as noncompetitive type. Under high concentration of 10-4 mol/L, the inhibition of Hg2+ on the enzyme is found to be largely of competitive type.
Key words: papain; Hg2+; kinetics; enzymic activity; secondary structure; irreversible modification
酶作为一种生物催化剂,已广泛地应用于工业生产的各个领域。木瓜蛋白酶是一种最常用的工业蛋白酶,在食品、医药、纺织等生产中广泛应用[1]。研究表明重金属通常是酶系统抑制剂,使酶的生产和使用受到极大的限制[2]。当前研究重金属对酶活性作用主要侧重于重金属与酶活性及其结构的关系上[3],而重金属作用下酶活性发生变化的过程和机理,以及酶动力学特征研究较少。若要在分子水平上了解重金属作用下酶的催化活性,探讨其本质,还需得到动力学研究的支撑,重金属作用下酶动力学参数可反映重金属、酶以及与底物的紧密程度和作用过程,阐明酶反应机理,进一步揭示酶与重金属间的内在联系。本文作者考察了Hg2+对木瓜蛋白酶的作用,研究Hg2+对木瓜蛋白酶构象的影响,从分子水平上研究其与木瓜蛋白酶之间相互作用机理。研究Hg2+作用下木瓜蛋白酶动力学,阐明其结构及其活性作用机理,揭示木瓜蛋白酶微观结构与催化性能的构效关系,为扩大木瓜蛋白酶的工业应用提供理论基础。
1 材料与方法
1.1 实验材料与仪器
木瓜蛋白酶[EC 3.4.22.2] (美国Sigma公司生产,纯度≥99%),半胱氨酸,酪蛋白 (上海伯奥生物科技有限公司生产),所用化学试剂均为分析纯。UV-2001型紫外分光光度计(日本岛津公司制造),Thermo公司Nicolet 380 FT-IR (分辨率 4 cm-1,扫描100次)。PE公司LS55型荧光光谱仪(激发波长288 nm,扫描速度为500 nm/min,扫描范围为310~400 nm)。将1.0 mL木瓜蛋白酶液分别与1.0 mL不同浓度的HgCl2溶液(0,10–4和10–6 mol/L)混合,加入8.0 mL的PBS缓冲溶液,45 ℃保温1 h,进行荧光光谱测定。
1.2 实验方法
(1) 精确称取HgCl2,用磷酸缓冲液(PBS,浓度0.1 mol/L和pH 7.0)配成一定浓度的HgCl2溶液。精确称取木瓜蛋白酶,用PBS液定容至浓度为1.0 mg/mL的酶液。称取2.0 g酪蛋白溶解于60 mL的PBS液中,加热煮沸,冷却,PBS液定容配成2.0 g/mL的酪蛋白溶液。将HgCl2溶液、酶液和酪蛋白溶液按1:1:3体积比混合反应,考察Hg2+对木瓜蛋白酶活性的影响。
(2) 酶活力测定参见文献[4]:取1.0 mg/mL的酶液1 mL与1 mL不同浓度Hg2+混合,40 ℃下保温10 min,再加入3 mL预热10 min的酪蛋白溶液,45 ℃反应30 min,加入10%三氯乙酸 (TCA) 溶液3.0 mL终止反应,振荡后45 ℃放置30 min,过滤后取上清液,测定UV,以原酶(纯酶)酶液为对照。木瓜蛋白酶的1个酶活力单位(U)定义:45 ℃,pH 7.0条件下,单位时间内水解酪蛋白产生l μg酪氨酸的酶量。相对酶活以未经Hg2+处理的酶酶活为100%计。
2 结果与讨论
2.1 Hg2+浓度对酶活的影响
考察不同浓度Hg2+对木瓜蛋白酶的酶活影响,结果见图1。由图1可知:当Hg2+浓度低于10-7 mol/L时,Hg2+对酶活力几乎无影响。10-6~10-7 mol/L Hg2+对酶有激活作用,10-6 mol/L Hg2+可使其相对酶活达到最高,为111.03%。当Hg2+浓度高于10-5mol/L时,Hg2+对酶活力具有抑制作用,当Hg2+的浓度大于10-4 mol/L时对酶的抑制作用达到最强,相对酶活降至6%左右。由上述结果可知:10-6 mol/L Hg2+对酶有弱激活作用,10-4 mol/L Hg2+对酶有强的抑制效应,Hg2+对木瓜蛋白酶作用表现出类似Hormesis效应的低促,高抑现象[5]。
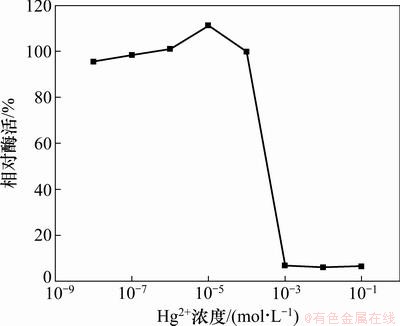
图1 Hg2+对木瓜蛋白酶的影响
Fig. 1 Effect of Hg2+ on papain activity
2.2 Hg2+作用下木瓜蛋白酶构象分析
酶分子活性与酶分子的构象紧密相关。为了进一步研究Hg2+对木瓜蛋白酶的抑制和激活机理,本文采用10-4和10-6 mol/L的Hg2+对木瓜蛋白酶进行处理,以纯酶为对照,将样品进行FT-IR和荧光光谱表征分析,探求Hg2+对木瓜蛋白酶构象的影响。
2.2.1 FT-IR分析
蛋白质红外光谱酰胺Ⅰ带峰主要是由蛋白质骨架中的羰基振动引起, 能够灵敏地反映蛋白质的构象及其变化。酰胺I带(1 700~1 600 cm–1)对蛋白质二级结构(α-螺旋、β-折叠、无规卷曲、转角结构)的变化最为敏感[6],故用酰胺I带分析Hg2+处理的木瓜蛋白酶二级结构差异。为了更好地分析其差异和得到详细的二级结构信息,利用二阶导数、去卷积和曲线拟合(高斯拟合)技术对样品酰胺Ⅰ带FT-IR图谱进行处理[7],结果见表1。
由表1可知:纯酶、10–6 mol/L Hg2+处理的酶与10–4 mol/L Hg2+处理的酶β-折叠都无太大差异,说明β-折叠结构较稳定,Hg2+处理对其二级结构并未产生太大影响。相对于纯酶(41.60%),10–6 mol/L Hg2+处理的酶α-螺旋含量增加了13.98%,说明酶的非极性残基增多;而10–4 mol/L Hg2+处理的酶α-螺旋含量减少了39.72%,说明酶的非极性残基减少。相对于纯酶(22.03%),10–6 mol/L Hg2+处理的酶β-转角含量减少了21.85%,而10–4 mol/L Hg2+处理的酶β-转角含量增加了83.34%。10–6 mol/L Hg2+处理的酶无规则卷曲含量增加了3.18%,而10–4 mol/L Hg2+处理的酶无规则卷曲含量减少了27.84%。
表1 木瓜蛋白酶酰胺I带的子峰位置、面积及二级结构归属
Table 1 Curve fitting analyses expressed as component bands, areas and assignments in amide I infrared bands of the secondary structures from papain
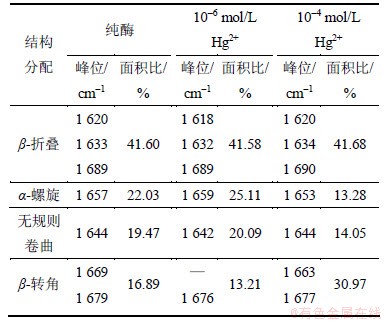
在蛋白质的二级结构中,α-螺旋和β-折叠结构具高度稳定性,其含量可用于衡量蛋白结构的稳定性,为蛋白质的有序结构;β-转角和无规卷曲为蛋白质的无序结构[8]。10–6 mol/L Hg2+处理的酶分子有序结构含量最高(66.69%),纯酶次之(63.63%),10–4 mol/L Hg2+处理的酶含量最低(54.96%),说明10–6 mol/L Hg2+处理的酶稳定性增加,而10–4 mol/L Hg2+处理的酶稳定性降低,而这些变化是由于α-螺旋结构的改变所致。另外,10–6 mol/L Hg2+处理的酶二级结构的各归属所占的含量与纯酶的相应归属含量的差异大大小于10–6 mol/L Hg2+处理的酶与纯酶的差异。无规则卷曲经常构成酶的活性部位[9],β-转角与酶的活性密切相关[10],它们对酶活力提高起至关重要的作用。10–6 mol/L Hg2+对木瓜蛋白酶有刺激作用,可能是由于无规则卷曲增加,β-转角减少,使得酶活性中心位结构更具有柔性、更合理,从而使其催化活性提高。反之亦然,10–4 mol/L Hg2+对木瓜蛋白酶有抑制作用。10–4 mol/L Hg2+对其二级结构β-转角和无规则卷曲的影响大大高于10–6 mol/L Hg2+的,表现为10–4 mol/L Hg2+的抑制效应大大高于10–6 mol/L Hg2+的激活效应。在低浓度下Hg2+对酶可产生过度补偿效应——激活效应,高浓度下为抑制效应的Hormesis现象[11]。综上所述,α-螺旋结构与木瓜蛋白酶的稳定性相关,其含量越高,结构越稳定。木瓜蛋白酶的β-转角和无规则卷曲与酶的活性密切相关,其酶的活性中心位可能是由β-转角和无规则卷曲决定,β-转角含量减少,无规则卷曲含量增加,有助于酶活提高,反之酶活降低。
2.2.2 木瓜蛋白酶荧光光谱分析
荧光光谱法是研究蛋白质与其他物质相互作用的极为有效的方法之一,可以提供大量蛋白质的构象变化信息[12]。图2所示为木瓜蛋白酶荧光光谱。从图2可知:10–6和10–4 mol/L Hg2+处理的酶荧光发射峰均为单峰,峰形基本相同,但最大荧光强度(Imax)和峰位(λmax)有异。与纯酶相比,10–6 mol/L Hg2+处理酶λmax蓝移2 nm,可能主是由于酶分子中α-螺旋含量增加(表1),酶分子内氢键和二硫键稳定更强[13];Imax增大,可能是由于Hg2+使酶分子中的色氨酸、酪氨酸残基处于更大的极性微区,酶与底物亲和力增强,酶活性增强。而10–4 mol/L Hg2+处理的酶λmax红移14 nm,是由于酶分子中α-螺旋含量减少(表1),酶二级结构稳定性降低;由于荧光猝灭,其Imax减弱,仅为纯酶的35.05%,可能是因为色氨酸、酪氨酸残基处于非极性微区,酶与底物亲和力减弱,酶活性降低。从图2可知:10–4 mol/L的Hg2+使酶λmax移位程度明显大于10–6 mol/L的Hg2+,说明后者的抑制作用大大高于前者的抑制作用。这些结构均与FT-IR分析结果一致(表1)。
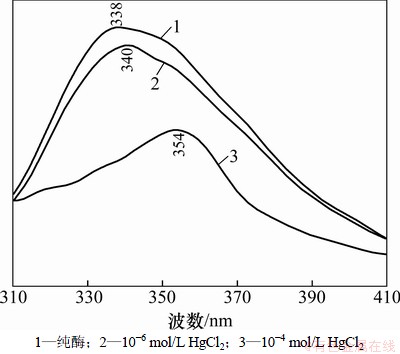
图2 木瓜蛋白酶荧光光谱
Fig. 2 Fluorescence emission spectra of papain
2.3 Hg2+作用下木瓜蛋白酶的反应动力学研究
2.3.1 木瓜蛋白酶活性不可逆动力学
Hg2+对木瓜蛋白酶表现出低促高抑的Hormesis现象,Hg2+作用于酶活性部位使酶的构象发生改变,Hg2+与酶的结合为不可逆反应,符合如下反应机制:
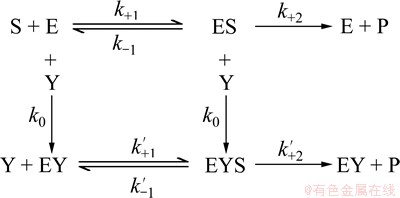
式中:E,S,Y,P,EY,ES和EYS分别表示酶、底物、Hg2+、产物及其相应的络合物;k0和k0′分别为Hg2+存在下游离酶的失活速率常数和结合酶ES的失活速率常数。根据单底物酶反应下邹氏酶活性不可逆动力学理论[14],设底物浓度[S]不随时间改变,酶与Hg2+结合后仍存在部分酶活,此时k+2和k+2′不为0,则t时刻的产物浓度表示为:
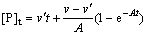 (1)
(1)
其中: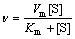 ;
;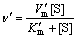 ;
;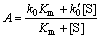 ;
;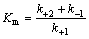 ;
;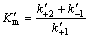 。
。
式中:A表示酶与Hg2+结合的表观速度常数;v和v′分别代表酶与Hg2+结合前后的反应速度,v>v′时,该反应为抑制过程;v<v′时,为激活过程;Km和Km′为米氏常数;Vm和Vm′为最大反应速度。当t→∞,即反应达到平衡时,终产物浓度不再随时间t变化而变化,达到最大值,记为[Pe][15],则:
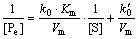 (2)
(2)
2.3.2 米氏常数的测定
在温度45 ℃,pH 7.0,酶浓度为1.0 mg/mL的条件下,分别用0,10–4和10–6 mol/L的Hg2+处理木瓜蛋白酶,测定不同酪蛋白质量浓度(0.2,0.4,0.6,0.8,1.0,1.6和2.0 mg/mL)下木瓜蛋白酶的酶促初速度。木瓜蛋白酶催化反应遵循于米氏方程,按Lineweaver -Burk双倒数图方法作图,结果如图3所示。与纯酶相比,10–6 mol/L Hg2+处理的酶Km减小,为纯酶的0.39倍,亲和力增大,Vm增加了9.2%;10–4 mol/L Hg2+处理的酶Km略有增加,为纯酶的1.05倍,亲和力减弱,Vm陡降了32.44%。从Vm的变化程度看:10–4 mol/L Hg2+处理的酶Vm显著下降(32.44%),而10–6 mol/L Hg2+的仅略有增加(9.2%),说明10–6 mol/L Hg2+处理的酶的激活效应远远低于10–4 mol/L Hg2+的抑制效应;从Km的变化来看:10–6 mol/L Hg2+处理的酶Km显著下降(0.39倍),而10–4 mol/L Hg2+的只是Km略有增加(1.05倍)。虽然10–6 mol/L Hg2+的Km的变化幅度比10–4 mol/L Hg2+的大,但前者对酶活性的影响显著比后者低。综合上述Hg2+对酶活影响(图1)及构象与酶活构效关系研究分析(表1和图2),可得出虽酶表面亲和力对酶活有影响,但酶活的改变主要取决于其活性中心位构象的变化,尤其是无规则卷曲和β-转角的变化。
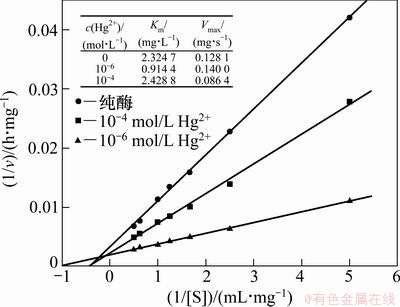
图3 木瓜蛋白酶的动力学参数
Fig. 3 Kinetic parameters of papain
2.3.3 Hg2+对木瓜蛋白酶动力学特征的影响
图4所示为不同酪蛋白质量浓度下,纯酶以及10–4和10–6 mol/L Hg2+处理的酶催化底物的水解反应。从图4可知:随着反应时间的延长,产物浓度趋近一个恒值。木瓜蛋白酶酶水解动力学符合邹承鲁关于单底物酶不可逆改变的动力学方程[14],即式(1)。纯酶水解反应中产物浓度随着时间的增加呈近似线性变化,10–6 mol/L Hg2+处理的酶水解反应呈激活酶促反应模式(图4(b)),而10–4 mol/L Hg2+处理的木瓜蛋白酶水解反应呈抑制酶促反应模式(图4(c))。木瓜蛋白酶的水解动力学符合式(1),将实验数据进行非线性最小二乘法的Exponential Linear函数拟合,模拟结果见表2。
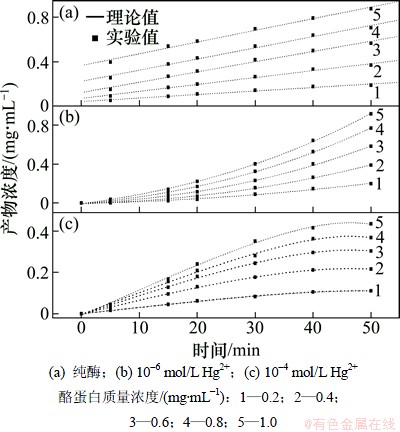
图4 产物浓度与时间的关系
Fig. 4 Relationship between product concentration and time
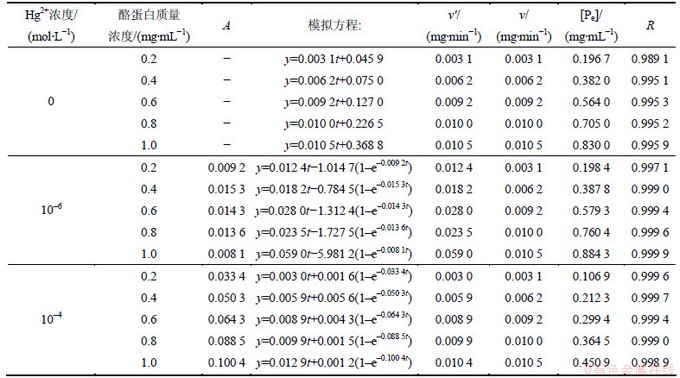
从动力学参数(表2)可知:式(1)的不可逆改变动力学模型的拟合理论数据与试验结果相符,相关性好(R≥0.99),该模型可以很好地解释Hg2+与酶结合的动力学规律。由式(2),以1/[Pe]对1/[S]作图(图5),得10–6 mol/L Hg2+处理酶的微观速度常数(R=0.999 9)k0=0.150 9和k0′=0.015 0,k0′≠k0,且k0比k0′大10倍;10–4 mol/L Hg2+处理酶微观速度常数(R= 0.999 4)k0=0.063 4和k0′=0.034 4,其k0′≠0,且k0比k0′大1倍。根据邹氏模型(表2),在相同底物浓度下Hg2+浓度越高,酶的表观反应速度ν和ν′越大,说明Hg2+的效应随其浓度的提高而增大。修饰剂与酶的竞争性、非竞争性以及反竞争性的关系可以根据底物对表观速度常数A的影响决定[16]。由表2可知:10–6 mol/L Hg2+处理的酶,ν<ν′,说明10–6 mol/L Hg2+对木瓜蛋白酶产生激活效应。A与[S]无关,即1/A与Km/(Km+[S])无线性关系,表明10–6 mol/L Hg2+与木瓜蛋白酶存在非竞争性不可逆激活关系。虽该浓度下k0′≠k0,k0比k0′大10倍,表明Hg2+(Y)与酶E结合生成EY速率远远大于Hg2+与ES结合生成EYS的。这些结果说明:低浓度下10–6 mol/L Hg2+对木瓜蛋白酶为非竞争性不可逆激活作用,Y与E结合不影响E与底物S结合,大部分Hg2+与酶的活性中心位以外的必需基团的激活位点结合,促使酶活性中心位结构更有利于与S结合,酶的活性增加。由于Hg2+为非专一性修饰剂,少量Hg2+与酶的活性中心位以外的抑制位点结合,继而和ES结合形成EYS不能进一步释放出产物,故表现出k0′≠k0,而Hg2+与必需基团的激活位点结合远远多于抑制位点(k0≈10k0′),从而表现出激活效应。10–4 Hg2+处理的酶ν>ν′,说明10–4 mol/L Hg2+对木瓜蛋白酶为抑制效应。A随[S]的增加而增大,即1/A与Km/(Km+[S])存在线性关系(R=0.993 8),表明10–4 mol/L Hg2+是木瓜蛋白酶竞争性不可逆抑制剂。该浓度下k0′≠0,且k0≈2k0′,表明Hg2+(Y)与酶E结合生成EY的速率大于Hg2+与ES结合生成EYS。这些结果说明:高浓度下10–4 mol/L Hg2+是木瓜蛋白酶竞争性不可逆抑制剂,Y与E结合生成EY,大量Hg2+与酶的活性中心位结合,与S竞争酶的活性中心,从而阻碍E与S的结合。同时少量Hg2+与酶的活性中心位以外的必需基团位点结合,继而和ES结合形成EYS而不能进一步释放出产物,故表现出k0′≠0。所以,10–4 mol/L Hg2+对木瓜蛋白酶是以竞争性不可逆抑制为主的混合型抑制。
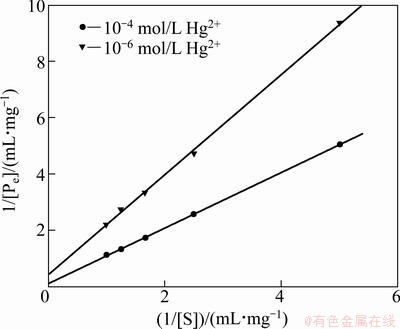
图5 1/[Pe]与1/[S]关系图
Fig. 5 1/[Pe] versus 1/[S]
表2 木瓜蛋白酶的动力学方程
Table 2 Kinetics equations of papain
3 结论
(1) 低浓度(10-6 mol/L)下Hg2+对酶有激活作用,高浓度(10-4 mol/L)下Hg2+对酶有抑制效应,Hg2+对木瓜蛋白酶作用表现出低促高抑的Hormesis现象。木瓜蛋白酶的β-转角和无规则卷曲与酶的活性密切相关,其酶的活性中心位可能是由β-转角和无规则卷曲决定,β-转角含量减少,无规则卷曲含量增加,有助于酶活提高,反之酶活降低。
(2) 通过研究Hg2+作用下木瓜蛋白酶水解酪蛋白的不可逆反应动力学,探讨Hg2+与木瓜蛋白酶结合动力学规律。邹氏酶活性不可逆改变动力学模型的拟合理论数据与本文试验结果相吻合,该模型可准确解释Hg2+与酶结合的动力学规律。低浓度下,10–6 mol/L Hg2+是木瓜蛋白酶的非竞争性不可逆激活剂,大部分Hg2+与酶的活性中心位以外的必需基团的激活位点结合,促使酶活性中心位结构更有利于与底物结合,酶的活性增加。高浓度下,10–4 mol/L Hg2+是木瓜蛋白酶竞争性不可逆抑制剂,大量Hg2+与酶的活性中心位结合,与底物竞争酶的活性中心,从而阻碍酶与底物的结合。少量Hg2+与酶的活性中心位以外的必需基团位点结合,继而和ES结合形成EYS而不能进一步释放出产物,所以10–4 mol/L Hg2+对木瓜蛋白酶是以竞争性不可逆抑制为主的混合型抑制。本文通过研究Hg2+作用下木瓜蛋白酶水解酪蛋白的不可逆反应动力学,从分子水平上阐明Hg2+与木瓜蛋白酶之间相互作用机理,揭示木瓜蛋白酶微观结构与催化性能的构效关系,从理论上为木瓜蛋白酶的工业实际应用提供依据。
参考文献:
[1] 贺枫, 卓仁禧, 刘立建, 等. 固定化木瓜蛋白酶的制备和性质研究[J]. 高分子学报. 2000(5): 637-640.
HE Feng, ZHUO Renxi, LIU Lijian, et al. Studies on the preparation and characterization of immobilized papain[J]. Acta Polymerica Sinica, 2000(5): 637-640.
[2] Zhao Y, Wang X, Qin Y, et al. Mercury(Hg2+) effect on enzyme activities and hepatopancreas histostructures of juvenile Chinese mitten crab Eriocheir sinensis[J]. Chinese Journal of Oceanology and Limnology, 2010, 28(3): 427-434.
[3] 洪法水, 王雪峰, 吴康, 等. 重金属离子对猪胰α-淀粉酶活性影响的作用机理研究[J]. 高等学校化学学报, 2001, 22(12): 1979-1983.
HONG Fashui, WANG Xuefeng, WU Kang, et al. Mechanism of heavy metal ions on α-amylase activity from porcine pancreas[J]. Chemistry Journal Chinese Universities, 2001, 22(12): 1979-1983.
[4] 何平, 黄卓烈, 初志战, 等. 乙腈对木瓜蛋白酶活性与构象的影响[J]. 上海交通大学学报, 2006, 24(3): 245-249.
HE Ping, HUANG Zhuolie, CHU Zhizhan, et al. Effect of a cetonitrile on activity and conformation of papain[J]. Journal of Shanghai Jiaotong University, 2006, 24(3): 245-249.
[5] 卢振伟, 李伟国, 赵大庆. 镧对小鼠肝脏中几种酶活性的影响[J]. 化学研究, 2002, 13(2): 1-4.
LU Zhenwei, LI Weiguo, ZHAO Dajqing. Effect of La3+ on the activity of several enzymes in mice liver[J]. Chemical Research, 2002, 13(2): 1-4.
[6] Zhao X , Chen F, Xue W, et al. FTIR spectra studies on the secondary structures of 7S and 11S globulins from soybean proteins using AOT reνerse micellar extraction[J]. Food Hydrocolloids, 2008, 22(4): 568-575.
[7] Choi E S M, Ma C Y. Structural characterization of globulin from common buckwheat (Fagopyrum esculentum Moench) using circular dichroism and Raman spectroscopy[J]. Food Chem, 2007, 102(1): 150-160.
[8] 吴黎明, 周群, 周骁, 等. 蜂王浆不同贮存条件下蛋白质二级结构的Fourier变换红外光谱研究[J]. 光谱学与光谱分析, 2009, 29(1): 82-87.
WU Liming, ZHOU Qun, ZHOU Xiao, et al. FTIR assessment of the secondary structure of proteins in royal jelly under different storage conditions[J]. Spectroscopy and Spectral Analysis, 2009, 29(1): 82-87.
[9] 王镜岩, 朱圣庚, 徐长法. 生物化学[M]. 北京: 高等教育出版社, 1989: 212.
WANG Jingyan, ZHU Shenggeng, XU Changfa. Biological chemistry[M]. Beijing: Higher Education Press, 1989: 212.
[10] 黎红晔, 张渝英, 董贻诚, 等. β-转角凝乳酶突变体的构建、表达和性质分析[J]. 生物工程学报, 1998, 14(1): 39-45.
LI Hongye, ZHANG Yuying, DONG Yicheng, et al. Construction, expression and characterization of calf chymosin β-turn mutants[J]. Chinese Journal of Biotechnology, 1998, 14(1): 39-45.
[11] Calabrese E J. Over compensation stimulation: A mechanism for hormetic effects[J]. Critical Reviews in Toxicology, 2001, 31(4/5): 425-470.
[12] 李向荣, 闫云辉. 荧光光谱法研究牛血清蛋白在盐酸胍体系中的变性[J].分析测试学报. 2011, 30(9): 1039-1043.
LI Xiangrong, YAN Yunhui. Denaturation study of bovine serum albumin indunced by guanidine ghloride with fluorescence spectroscopy[J]. Journal of Instrumental Analysis, 2011, 30(9): 1039-1043.
[13] 郭明, 庞铄权, 张璐颖. 重金属镉离子人工合成抗原的荧光特性分析[J]. 分析化学研究报告, 2009, 37(7): 1009-1013.
GUO Ming, PANG Shuoquan, ZHANG Luying. Detection of artifical antigen for Cd(Ⅱ) by fluorescence spectroscopy[J]. Chinese Journal of Analytical Chemistry, 2009, 37(7): 1009-1013.
[14] 邹承鲁, 赵康源. 酶活性不可逆改变动力学理论研究[J]. 自然科学进展, 1991, 1(1): 26-39.
TSOU Chenlu, ZHAO Kangyuan. Theory on the kinetics of irreversible modification of enzymic activity[J]. Progress in Natural Science, 1991, 1(1): 26-39.
[15] 黄守勤, 颜清, 林沁英, 等. 酶活性不可逆改变的底物保护动力学[J]. 厦门大学学报: 自然科学版, 1992, 31(1): 425-429.
HUANG Shouqin, YAN Qing, LIN Qingying, et al. Kinetic studies on the protection of enzyme activity by substrate during irreversible modification[J]. Journal of Xiamen University: Natural Science, 1992, 31(1): 425-429.
[16] 邹承鲁. 酶活性不可逆改变的动力学I[J]. 生物化学与生物物理学报, 1965, 5(4): 398-408.
TSOU Chenlu. Kinetic of irreversible modification of enzyme activity. I: The effect of substrate on the rate of binding between an enzyme and a modifier[J]. Acta Biochimica et Biophysica Sinica, 1965, 5(4): 398-408.
(编辑 杨幼平)
收稿日期:2012-12-05;修回日期:2013-03-17
基金项目:湖南省科技厅计划项目(2011SK3138); 湖南省研究生科研创新项目(CX2011B265); 湘潭大学大学生创新项目(2012年)
通信作者:曾虹燕(1963-),女,湖南益阳人,博士,教授,博士生导师,从事生物化工研究;电话:0731-58298175;E-mail:hyzeng@xtu.edu.cn