Slurry wear characteristics of zinc-based alloys: Effects of sand content ofslurry, silicon addition to alloy system and traversal distance
来源期刊:中国有色金属学报(英文版)2009年第2期
论文作者:B. K. PRASAD O.P. MODI
文章页码:277 - 286
Key words:zinc-based alloys; slurry wear behaviour; material removal mechanisms; erosion-corrosion-abrasion; microstructure- property correlations
Abstract: This investigation deals with the observations pertaining to the effects of specimen and slurry compositions as well as traversal distance on the slurry wear response of a zinc-based alloy. The composition of the alloy was altered by adding 4% silicon to it. The slurry composition was varied through changing the concentration of the sand particles in the range of 0-60% that were suspended in the (liquid) electrolyte. The electrolyte contained 4 g sodium chloride and 5 mL concentrated sulphuric acid dissolved in 10 L of water. The slurry wear tests were conducted at a speed of 7.02 m/s over the traversal distance range of 15-500 km. The wear rate increased initially with traversal distance, attained a maximum and decreased thereafter irrespective of the specimen and test environment. However, the wear rate peaks were less prominent in the liquid plus sand environments than the liquid-only medium. Further, the wear rate peak in the liquid-only medium appeared at a shorter traversal distance than the one in the sand containing slurries. Addition of sand particles to the electrolyte reduced the wear rate of the samples to 5%-15% depending on the sand concentration of the slurry. Moreover, intermediate (40%) sand content led to a maximum wear rate when compared with in the liquid plus sand media. However, this maximum was still less than in the liquid-only medium. The silicon containing alloy suffered from higher wear rates than the silicon free alloy samples when tested in the liquid-only medium. On the contrary, the trend reversed in liquid plus 20% and 40% sand environments whereas a mixed response was noted in the slurry containing 60% sand. In the latter case, the presence of silicon proved deleterious initially while an opposite trend was observed at longer traversal distances. The wear response of the samples was discussed in terms of specific features of their microconstituents like silicon and the predominant material removal mechanism in a given set of experimental conditions. The observed behaviour of the alloys was also substantiated further through the characteristics of their affected surface and subsurface regions.

B.K. PRASAD, O.P. MODI
Advanced Materials and Processes Research Institute (CSIR), Bhopal-462026, India
Received 16 April 2008; accepted 28 August 2008
Abstract: This investigation deals with the observations pertaining to the effects of specimen and slurry compositions as well as traversal distance on the slurry wear response of a zinc-based alloy. The composition of the alloy was altered by adding 4% silicon to it. The slurry composition was varied through changing the concentration of the sand particles in the range of 0-60% that were suspended in the (liquid) electrolyte. The electrolyte contained 4 g sodium chloride and 5 mL concentrated sulphuric acid dissolved in 10 L of water. The slurry wear tests were conducted at a speed of 7.02 m/s over the traversal distance range of 15-500 km. The wear rate increased initially with traversal distance, attained a maximum and decreased thereafter irrespective of the specimen and test environment. However, the wear rate peaks were less prominent in the liquid plus sand environments than the liquid-only medium. Further, the wear rate peak in the liquid-only medium appeared at a shorter traversal distance than the one in the sand containing slurries. Addition of sand particles to the electrolyte reduced the wear rate of the samples to 5%-15% depending on the sand concentration of the slurry. Moreover, intermediate (40%) sand content led to a maximum wear rate when compared with in the liquid plus sand media. However, this maximum was still less than in the liquid-only medium. The silicon containing alloy suffered from higher wear rates than the silicon free alloy samples when tested in the liquid-only medium. On the contrary, the trend reversed in liquid plus 20% and 40% sand environments whereas a mixed response was noted in the slurry containing 60% sand. In the latter case, the presence of silicon proved deleterious initially while an opposite trend was observed at longer traversal distances. The wear response of the samples was discussed in terms of specific features of their microconstituents like silicon and the predominant material removal mechanism in a given set of experimental conditions. The observed behaviour of the alloys was also substantiated further through the characteristics of their affected surface and subsurface regions.
Key words: zinc-based alloys; slurry wear behaviour; material removal mechanisms; erosion-corrosion-abrasion; microstructure- property correlations
1 Introduction
High strength zinc-based alloys generally comprising substantially large quantities of Al (>8%) and 1%-3% Cu have been established as a potential material system for use in a variety of engineering applications encountering wear[1-3]. In many applications, corrosive environments with suspended solid particles are also encountered in practice in addition to wear by the (zinc-based) alloys. For example, mining machinery components form an important category of applications wherein there exists the possibility of the components coming in contact with mine water plus sand/soil particles during service. Further, in applications of the zinc-based alloys as coatings and sacrificial anodes to protect steel structures against atmospheric corrosion also, wear takes place in corrosive environments[4-8].
Zinc-based alloys have been shown to be more effective as protective coatings and sacrificial anodes than pure zinc in more corrosive environments[9-11]. It has been observed that the corrosion resistance of zinc-based alloys increases with Al content[12] while addition of Si to the alloy system has been proved to be still more beneficial in this context[9,11]. Studies suggest that Zn-Al alloys behave in a manner similar to that of pure Zn from corrosion resistance standpoint since Al remains practically unaffected[13]. However, the presence of iron leads to higher chemical activity of the alloy system[13]. Conflicting observations have been made as far as the role of reinforcement particles in controlling the corrosion resistance of the zinc-based alloys is concerned. For example, the presence of SiC reinforcement in the alloy matrix has been noted to improve the corrosion resistance of the alloy system [14-16], as evinced by reduced open circuit potential [14]. Better response of the composite has been attributed to the passive/inert nature of the SiC particles that replace some fraction of the more active metallic material surface exposed to the corrosive environment [14]. On the contrary, a reversal in the trend has also been noted in view of severe dispersoid/matrix interfacial attack[17-19]. From microstructure point of view, corrosion resistance improves with increasing secondary dendritic arm spacing in hypereutectic Zn-Al alloys [20-21] while the trend reverses in the case of hypoeutectic alloys[20]. The corrosion process in Zn-Al alloys has been shown to initiate in the Al rich region in the interdendritic area[9] while the Zn rich phase is more susceptible to corrosion[14].
High strength zinc-based alloys in general suffer from shortcomings like dimensional instability and inferior elevated temperature mechanical properties that limit their use to slow moving applications operating below 120 ℃[1-3]. Recently, a few modified versions of zinc-based alloys have been developed through the addition of silicon, showing potential to reduce the mentioned shortcoming of the high strength alloys [22-41]. Si in this case imparts improved physical, mechanical and wear properties to the alloy system under specific conditions[9-11,22-31,41].
Wear being a surface phenomenon is a complex process of material removal. This is evident from a variety of material and test parameters which greatly control the wear behaviour of materials[29-31,42-58]. The degree of complexity increases further in the presence of a chemical (corrosive) environment, more so in the presence of suspended solid mass. As far as slurry wear response of materials is concerned, material related controlling factors include nature, type, size, shape, content, chemical reactivity to the test environment and hardness of their various microconstituents; the nature of phase/matrix interfacial regions; work hardening capability; cracking tendency, compactness and stability of the reaction products on the affected surface etc. [42,44-46,48-49,53-56,58]. Experimental parameters affecting the slurry wear characteristics include the nature (pH) of the electrolyte, the characteristics and features of the suspended solid (erosive) particles in the electrolyte, traversal distance, radial distance, speed, angle of attack etc.[42-56,58]. It has been observed that no direct relation exists between the parameters and the response of materials, and even mixed influence has been observed in many instances. For example, the performance of materials deteriorates initially with increasing speed, traversal distance and solid content in the medium but the trend becomes opposite at still higher values of the parameters[42,50,58-60]. Also, increasing radial distance, angle of inclination/attack and interfacial attack accelerates the severity of damage[42-58]. Further, the addition of suspended solid mass to the electrolyte has been found to deteriorate the response of materials [45,47] while opposite effects have also been observed [42,50,58-60]. Changing severity of surface damage has been attributed to the predominance of one damage mechanism over the other[42,50,58-60]. The complex nature of the influence of controlling parameters on the wear behaviour of materials is in view of the fact that in addition to the wear process, chemical effects also come into picture and have a synergistic influence on the overall wear response of the samples[11,55-57,61-65]. For example, both erosion and corrosion have been noticed to accelerate each other’s negative influence[66]. Corrosion accelerates erosion[61-63] through surface roughening[11,63] since the severity of erosion is sensitive to the angle of attack[60]. Also, removal of the work hardened layer (produced by the impact of eroding particles) due to corrosion further enhances erosion [11,63-65]. On the contrary, erosion enhances corrosion through the removal of the surface deposits, surface roughening and increase of local turbulence[11] while the work hardened layer produces a reverse effect. Entrapped erosive particles decrease the wear rate. The severity of further material loss may increase due to preferential interfacial attack or decrease as a result of reduced effective area of the corroding metallic surface.
An appraisal of the available information indicates that some studies have been carried out pertaining to the corrosion characteristics of zinc-based alloys in different media[4-16,20-21,55,61-64,66-74] while very limited investigations deal with the response of the zinc-based alloys in slurries[42,58-60]. The highly sensitive nature of the wear response of materials in corrosive environments and synergistic effects of parameters involved therein[11,55-57,61-65] suggest the need to assess the corrosive wear characteristics of zinc-based alloys in order to widen the range of their applications. The influence of silicon on the wear behaviour of the alloy system in corrosive environments adds to the significance of the studies further in view of its application potential.
In view of the above, an attempt has been made in this study to analyze the response of a (high strength) zinc-based alloy in various test environments over a range of traversal distances. The influence of adding 4% silicon to the alloy system on its wear characteristics has also been studied in identical test conditions. The wear behaviour of the samples observed in various test conditions has been explained on the basis of specific roles played by the silicon particles and the predominant nature of different operating mechanisms of material removal in view of the changing nature of the test environment. Analyses of the features of affected surfaces and subsurface regions further substantiated the observed response of the samples.
2 Experimental
2.1 Alloy preparation and determination of properties
The experimental zinc-based alloys (Table 1) were prepared by liquid metallurgy route. The alloy melts were solidified in the form of 20 mm diameter, 150 mm long cylindrical castings using permanent moulds. Hardness and density measurements were carried out on 15 mm diameter and 15 mm thick samples that were cut from the castings and machined well. The samples were then polished as per standard metallographic techniques for the determination of their hardness and density properties. Density of the samples was determined by water displacement technique. A Mettler microbalance with a precision level of 0.01 mg was used for weighing the samples in air and water for computing the density. Hardness measurements were made using a Vickers’ hardness tester at an applied load of 294 N. The reported values of density and hardness represent an average of five observations, the range of variation being ±3%. Tensile tests were conducted on round samples having 4 mm gauge diameter and 20 mm gauge length. The apparatus used for conducting the tensile tests was an Instron make universal testing machine while the strain rate employed for the purpose was 4.6×10-3 s-1.
Table 1 Chemical compositions of experimental zinc-based alloys
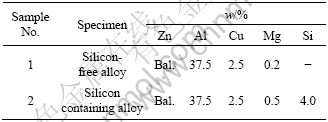
2.2 Specimen preparation and microscopy
Specimens (15 mm in diameter, 10 mm in thickness) for microstructural observations were prepared by polishing them metallographically and etching with diluted aqua regia. For conducting slurry wear tests, the samples (15 mm in diameter, 10 mm in thickness) were metallographically polished. Affected surfaces after the wear tests were cleaned thoroughly with acetone for their analysis. Transverse sections were cut from the affected surfaces, mounted in polyester resin, polished metallographically and etched with diluted aqua regia for analyzing the subsurface regions.
Microstructural characterization was carried out using a Leitz optical microscope whereas the affected surfaces and subsurface regions were studied with the help of a scanning electron microscope (SEM). The specimens were mounted on brass studs and sputtered with gold prior to their SEM examination.
2.3 Slurry wear tests
Slurry wear tests were conducted on metallographically polished (15 mm in diameter, 10 mm in thickness) samples by sample rotation technique. A schematic representation of the test apparatus is shown in Fig.1. Samples fixed on a non-conducting disc were rotated at a speed of 7.02 m/s for various traversal distances (15-500 km) in a container having the slurry. The composition of the test environment was changed by adding sand particles (212-300 mm) in varying quantities (0-60%) to an electrolyte. The electrolyte was prepared by dissolving 4 g sodium chloride and 5 mL concentrated sulphuric acid in 10 L of water, a composition conforming to mine water. The specimens were cleaned well with acetone prior to and after testing and weighed using a Mettler microbalance with a precision level of 0.01 mg. Wear rate was computed by mass loss measurement. An average of three observations was reported in this study.
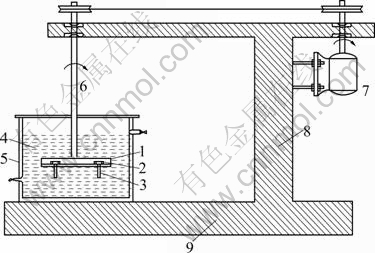
Fig.1 Schematic representation of slurry wear tester: 1—Disc; 2—Sample holder; 3—Sample; 4—Slurry medium; 5—Double wall container; 6—Spindle; 7—Driving motor; 8—Column; 9—Machine base
3 Results
3.1 Microstructural observations and properties
Fig.2 shows the microstructure of the alloys. The sample without silicon reveals dendritic structure comprising primary α, eutectoid α+η and ε (Fig.2(a), regions marked by A, B and arrow respectively). Alloying with silicon led to the formation of discrete particles of silicon (Fig.2(b), region marked by C). Table 2 shows the density, hardness and tensile strength and elongation of the samples. The silicon containing alloy attains less density but higher hardness as compared with that of the silicon-fee alloy samples. Further, the presence of Si in the samples also decreases their strength and elongation.

Fig.2 Microstructural features of silicon-free (a) and silicon containing alloys (b) (A: Primary α; B: α+η; Arrow: ε; C: Silicon particles)
Table 2 Properties of experimental zinc-based alloys

3.2 Slurry wear response
Wear rate of the samples was plotted as a function of traversal distance (Figs.3 and 4) and sand content for a typical traversal distance of 500 km (Fig.5). The influence of adding 4% Si to the alloy system on the wear rate of the samples is evident in the figures. The wear rate increases with traversal distance, attains the maximum and decreases thereafter at longer traversal distances (Figs.3 and 4). The rate of initial increase in wear rate with distance is substantially larger; the wear rate peak becomes sharper and higher; and the peak is observed at shorter distances in the liquid-only medium as compared with that in liquid plus sand environments (Figs.3 and 4). Addition of silicon to the alloy system increases the wear rate when tests are conducted in the liquid-only medium (Fig.3). A reverse trend is observed in the liquid plus 20%-40% sand environments whereas a mixed response is noted in the medium containing 60% sand (Fig.4). In the latter case, the silicon containing alloy performed better than the silicon-free alloy samples at longer distances whereas an opposite trend is followed initially. Presence of the sand particles in the test environment decreases the wear rate of the samples to 5%-15% depending on the sand content as compared with that in the liquid-only medium (Figs.3, 4 and 5). Moreover, a comparison of the wear response of the samples in the liquid plus sand slurries suggests the intermediate (40%) sand content to cause higher wear rate than the remaining (20% and 60%) sand contents in the medium.
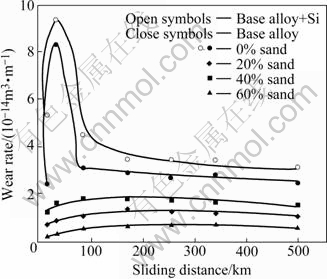
Fig.3 Wear rate plotted as function of travel distance for silicon-free alloy in various test environments and its comparison with silicon containing alloy in liquid-only medium

Fig.4 Wear rate plotted as function of travel distance for alloys with and without silicon tested in liquid plus sand environments
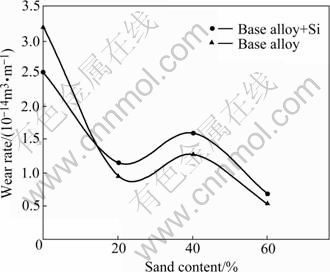
Fig.5 Wear rate of silicon containing and silicon free zinc-based alloys plotted as function of sand content of slurry for typical travel distance of 500 km
3.3 Affected surfaces
Fig.6 shows affected surfaces of the alloy without silicon in various test environments. Corrosive attack of the liquid-only medium causing the formation of pits on the specimen surface is evident in Fig.6(a) (region marked by single arrow). The severity of attack reduces considerably when tests are carried out in liquid plus 40% sand slurry (Fig.6(b)) over that in the liquid-only medium (Fig.6(a)). A typical indentation mark on the specimen surface in this case is evident in Fig.6(b) (region marked by A). A magnified view clearly reveals the pits and indentation marks (Fig.6(c), regions marked by single arrow and A, respectively). Abrasion grooves are observed on the affected surfaces of the samples tested in the slurry containing 60% sand (Fig.6(d), region marked by double arrow).

Fig.6 Affected surfaces of silicon-free alloy after testing for 500 km in liquid-only medium (a), 40% sand slurry (b, c), and 60% sand slurry (d) (Single arrow: Pits; A: indentation mark; Double arrow: Abrasion grooves)
Fig.7 represents affected surfaces of the alloy containing silicon. The influence of test environment and travel distance on the nature of surface damage is evident in the figure. The attack of the liquid-only environment initiated in the form of fine pits in different locations of the specimen surface (Fig.7(a), region marked by single arrow). The severity of attack of the medium increases with increasing travel distance (Fig.7(b)). Silicon particle/matrix interfacial attack on the specimen surface by the medium is also noted (Fig.7(c), region marked by double arrow). The severity of attack by the test environment reduces in the presence of the (40%) suspended sand particles (Figs.7(d) vs (c)) while abrasion grooves are observed in the case of testing the alloy in liquid plus 60% sand slurry (Fig.7(e), region marked by triple arrow).
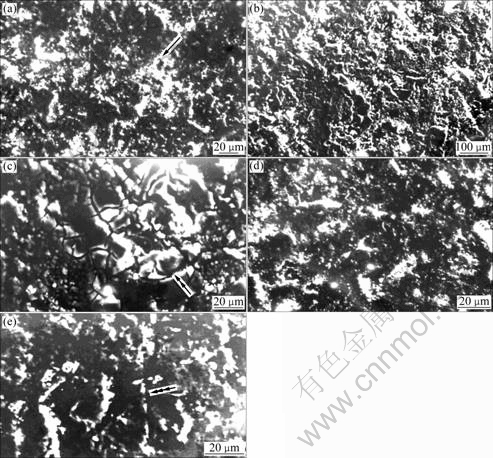
Fig.7 Affected surfaces of silicon containing alloy after testing in liquid-only medium (a)- (c), 40% sand slurry (d), 60% sand slurry for (a) 15 km, and 500 km (b)-(e) (Single arrow: Pits; Double arrow: Silicon particle/matrix interfacial attack; Triple arrow: Abrasion grooves)
3.4 Subsurface characteristics
Features of subsurface regions of the samples are shown in Fig.8. The regions marked by A and single arrow in Fig.8(a) represent an indentation mark and microcracking around the indented region, respectively. A typical sand particle entrapped in such an indented region is shown in Fig.8(b) (region marked by B). Microcracking of the sand particles was also observed (Fig.8(b), region marked by double arrow). Silicon particle/matrix interfacial attack below the affected surface (top portion) is evident in Fig.8(c) (region marked by triple arrow).
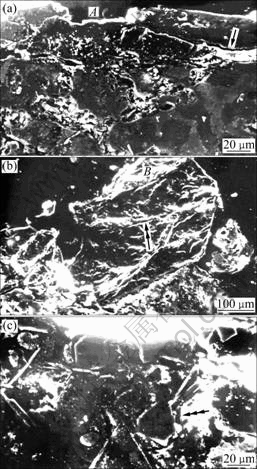
Fig.8 Subsurface regions of silicon-free (a, b) and silicon containing alloys (c) (A: Indentation mark; Single arrow: Microcracks around indented region; B: Typical sand particle entrapped in indented region; Double arrow: Microcracks in sand particle; Triple arrow: Silicon particle/matrix interfacial attack)
4 Discussion
From microstructural considerations, the Al rich α and zinc rich η phases are essentially solid solutions of Zn and Al in each other and basically soft in nature [23-25,31,34,36, 76]. Further, the ε phase is somewhat harder than the α and η phases and offers wear resistance [23-25,30,33,35,75]. As far as Si particles are concerned, they are the hardest amongst all the four phases. From hardness, strength and wear resistance points of view, the four phases could be graded as Si>ε>α>η [23-25,30,33,35,75]. The same gradation is also valid for the phases from corrosion resistance point of view. This could be conceived keeping in mind the place of the major constituents, i.e. Si, Cu, Al and Zn in Si, ε, α and η respectively, in the electrochemical series that suggests Si>Cu>Al>Zn in terms of the degree of electrochemical passivation[76]. Accordingly, the overall response of the specimens is controlled by the properties like hardness, strength and corrosion and wear resistance offered by the various microconstituents of the samples and the predominance of one set of characteristics of the constituent phases over the other producing a reverse effect[12,23-40,42,58,75]. The nature of the test environment is expected to play an important role in this context[42,58-60,75]. Higher hardness and less density of the Si containing zinc-based alloy than that of the Si free alloy samples could be attributed to the light mass and high hardness characteristics of Si [23-25,30,33,35, 75]. On the contrary, a reduction in the strength and elongation of the alloy system in the presence of Si (Table 2) could be attributed to the enhanced crack sensitivity[23-25,30,33,35,75]. It has been suggested that Si has negligibly small solid solubility both in Al and Zn[77]. This leads to the generation of discrete particles of Si in the alloy matrix (Fig.2(b), region marked by C). In such cases, interfacial regions become preferential sites for the nucleation followed by propagation of cracks in the material system[78-79] during tensile testing that ultimately leads to inferior tensile strength and ductility in the Si containing zinc-based alloy as compared with the samples without Si (Table 2).
Slurry wear testing by sample rotation technique involves the rotation of specimens fixed on a disc in the test environment. In the event of rotation in liquid alone, surface damage to the specimen is caused by the chemical attack and impinging action of the droplets of the medium. Droplets are formed due to the turbulence created by the rotating specimens in the medium. In case the medium contains suspended solid particles, damage due to the particles also takes place on the specimen surface. The damage occurs in the form of impingement of the solid particles on the exposed surface, i.e. erosion when their concentration in the medium is relatively low [45,50,58]. Another form of surface damage takes place through the sliding action of the suspended particles, i.e abrasion in view of their restricted mobility due to high concentration in the medium[50,58]. Entrapment of the impinging particles in the indented regions also occurs.
Damage to the rotating specimen surface by the medium initiates in the form of fine pits[42-48,58]. Growth of the pits takes place, leading to the formation of deep craters as the test progresses. By this time, reaction products are deposited inside/around the pits[42-48,58]. Accordingly, the deposited mass decreases the severity of attack by the medium by way of reducing the extent of penetration of the environment to the metallic surface. Evolution of hydrogen in the crevices/pits also produces a similar effect[42-48,58]. The reaction products are removed due to the impinging action of the medium especially when their mass/thickness exceeds a limit allowing the exposure of fresh metallic surface to the medium. Thus, the formation and removal of reaction products take place almost continuously in quite succession during the tests. The role of a reaction/corrosion product in controlling the wear behaviour of materials in slurry depends on its compactness (inverse of permeability) and adhesiveness with the substrate surface. The degree of compactness of a corrosion product in turn has been found to be dependent on the nature, shape, size and orientation of the particles therein[7]. Further, the higher the compactness and adhesiveness of the reaction product are, the less the severity of further corrosion would be due to the reaction product. Thus, removal of a corrosion product/passive layer due to erosive and/or corrosive action could be detrimental or beneficial depending on the degree of its compactness.
Coming to the composition of the test environment, it may be noted that the liquid comprises of sulphate and chloride ions which are quite corrosive in nature. Addition of sand particles to the liquid (maintaining the same volume fraction of the slurry) decreases the effective volume fraction of the (corrosive) liquid, thereby reducing the severity of its corrosive action. Increasing pH of the environment from 1.93 for the liquid-only medium to 3.64, 6.85 and 7.12 for the environments containing 20%, 40% and 60% sand, respectively, further substantiates the reducing corrosivity of the medium in the presence of the sand particles. However, the presence of the (sand) particles produces impinging action, causing erosive damage in view of their mobility in the rotating liquid[42-48,58]. The severity of erosion increases with the content of the suspended solid mass up to a critical value in the liquid. Beyond the critical content, the suspended solid particles enjoy only a limited freedom in the medium and rather experience sliding action against the specimen surface causing abrasion[42,50,58-59,75].
Initial increase in wear rate with distance (Figs.3 and 4) could be attributed to the increasing severity of attack by the environment (Figs.7(b) vs (a)). Wear rate peak corresponds to the formation of deep craters[42-48, 58-59,75]. A decrease in wear rate beyond the wear rate peak is due to the deposition of reaction products in/around the affected regions[42,50,58-59,75]. Entrapment of suspended sand particles in the indented regions (Fig.8(b), region marked by B) may be partially responsible for the decrease in wear rate in liquid plus sand environments over that in the liquid-only medium (Figs.3, 4 and 5).
Higher wear rate of the silicon containing alloy as compared with the silicon-free alloy samples in the liquid-only medium (Fig.3) in spite of less corroding nature of the silicon particles present therein could be owing to the predominant silicon particle/matrix interfacial attack[14] by the environment (Fig.7(c), region marked by double arrow, and Fig.8(c), region marked by triple arrow). On the contrary, better wear performance of the samples alloyed with silicon than the ones without the element in liquid plus sand environments (Fig.4) is due to the resistance offered by the silicon particles to the softer matrix against the destructive action of the environments[14,17-19]. Lower wear rate of the silicon-free alloy in the 60% sand environment at shorter travel distances (Fig.4) can be attributed to more firmly sticking of the reaction products on the affected surfaces[7] in view of better ductility and less hardness of the (silicon-free) alloy (Table 2). The sticking mass appears to offer resistance against the (abrasive) action of the medium causing improved wear response of the silicon-free alloy as compared to that of the one with silicon (Fig.4).
Maximum wear rate in the liquid-only medium (Figs.3 and 4) suggests that the dominant wear mechanism causing material loss is corrosion (Figs.6(a), 7(a)-(c) and 8(c)). A reduction in the wear rate in spite of the additional (erosive and/or abrasive) damage (Figs.6(b)-(d), 7(d) and (e), and 8(a) and (b)) by the suspended solid mass in the medium indicates that the additional (abrasive and/or erosive) damage by the (suspended) solid particles is less detrimental than the reduction in the (corrosive) attack of the medium in view of its decreasing volume fraction in the test environment. Increasing wear rate of samples due to the increasing solid content from 20% to 40% can be due to a greater severity of the impinging action of the solid mass causing erosion of the specimen surface (Figs.6(c) and 8(a), regions marked by A). On the contrary, a reduction in wear rate in the liquid plus 60% sand medium over the one in 40% sand slurry (Fig.5) can be owing to the dominating abrasive action of the sand particles on the specimen surface. It may be noted that abrasive action of the environment leading to the generation of grooves (Fig.6(d), region marked by double arrow, and Fig.7(e), region marked by triple arrow) is less detrimental in terms of material loss than their impinging action wherein large craters are formed (Fig.6(b), region marked by A, and Figs.8(a) and (b), regions marked by A and B).
An appraisal of the observations made in this investigation suggests significant effects of the presence of silicon in the alloy system on slurry wear response. Further, the nature and severity of the influence are dependent on the sand content of the test environments. Addition of sand particles to the test medium causes the wear rate to decrease. Intermediate sand content leads to maximum wear rate when compared with in the sand slurries. However, this maximum is less than that in the liquid-only medium. The predominant operating wear mechanism also changes with the sand concentration of the medium from corrosion in the liquid-only environment to corrosion-assisted-erosion in the slurry containing up to 40% sand and to corrosion-assisted- abrasion in the 60% sand slurry.
5 Conclusions
1) Wear rate increases initially with traversal distance, attains the (wear rate) peak and decreases thereafter at still longer traversal distances. The wear rate peak is quite prominent when the tests are conducted in the liquid-only medium; the peaks are rather shallow in the sand slurry and less defined in some cases. Further, the peaks are observed to reach at shorter distances in the liquid-only medium than those in the sand slurries.
2) The presence of Si in the alloy system proves beneficial by offering decreased wear rate in general when the tests are conducted in the liquid plus sand slurries. On the contrary, the Si containing alloy exhibits higher wear rates as compared to that of the silicon free alloy in the liquid-only medium.
3) Maximum wear rate of the samples is noted in the liquid-only medium. The presence of suspended sand particles in the test environment leads to a reduced wear rate irrespective of the alloy composition, the degree of reduction in the wear rate being to the extent of 5%-15% depending on the sand content of the slurry. A comparison of the wear behaviour of the samples in the sand slurries shows that the intermediate (40%) sand content leads to a maximum wear rate. However, this maximum wear rate is less than that in the liquid-only medium.
4) Corrosion is the dominant mechanism of material removal in this investigation, erosion and abrasion playing a secondary role. The predominance of one wear mechanism over the other is responsible for the changing behaviour of the samples with the composition of the test environment in terms of sand content. Also, the dominant and opposite effects of factors like silicon particle/matrix interfacial attack and (corrosion/erosion/abrasion) resistance offered by the silicon particles to the alloy system over each other are responsible for the varying wear response of the alloys in various test environments.
References
[1] Calayag T S. Zinc alloys replace bronze in mining equipment bushings and bearings [J]. Mining Engineering, 1983, 36: 727-728.
[2] Pratt G C. Materials for plain bearings [J]. International Materials Review, 1973, 18: 1-27.
[3] Kubel E J. Expanding horizons for ZA alloys [J]. Advanced Materials and Processes, 1987, 132: 51-57.
[4] Rausch W. Chemical surface treatment of steel coated with zinc and zinc alloys [C]// Proceedings of the International Conference of Zinc and Zinc Alloy Coated Steel Sheet (GALVATECH). Tokyo, Japan: The Iron and steel Institute of Japan, 1989: 199-201.
[5] Shin J C, Lee Y P, Choi K D, Jun J H. Proceedings of the International Conference of Zinc and Zinc Alloy Coated Steel Sheet (GALVATECH), Amsterdam, The Netherlands, Sept. 8-10, 1992.
[6] Wallinder I O, Leygraf C, Karlen C, Heijorick D, Janssen C R. Atmospheric corrosion of zinc-based materials: Run off rates, chemical speciation and ecotoxicity effects [J]. Corrosion Science, 2001, 43: 809-816.
[7] Ishikawa T, Ueda M, Kandori K, Nakayama T. Air permeability of the artificially synthesized Zn-Al-Mg alloy rusts [J]. Corrosion Science, 2007, 49: 2547-2556.
[8] Tachibana K, Morinaga Y, Mayuzumi M. Hot dip fine Zn and Zn-Al alloy double coating for corrosion resistance at coastal area [J]. Corrosion Science, 2007, 49: 149-157.
[9] Moeira A R, Panossian Z, Camargo P L, Moeira M, de Silva I C, de Carvalho J E R. Zn/55Al coating microstructure and corrosion mechanism [J]. Corrosion Science, 2006, 48: 564-576.
[10] Dalledone E, Barbosa M A, Wolymec S. Zinc-55%Al-1.6%Si coating compared with zinc coating [J]. Materials Performance, 1995, 34(7): 24-28.
[11] Allegra L, Dutton R J, Humayun A. Proceedings of the 1st Intergalva Conference. Munich, Germany, 1985: A/1-A/6.
[12] Chen W, Liu Q, Zhu L, Wang L. A combinatorial study of the corrosion and mechanical properties of Zn-Al material library fabricated by ion beam sputtering [J]. Journal of Alloys and Compounds, 2008, 459: 261-266.
[13] Dafydd H, Worsley D A, McMurray H N. The kinetics and mechanism of cathodic oxygen reduction on zinc and zinc-aluminium alloy galvanized coatings [J]. Corrosion Science, 2005, 47: 3006-3018.
[14] Pruthviraj R D, Krupakara P V, Nagaswarupa H P. Open circuit potential studies of ZA-27/SiC MMCs in acid choloride media [J]. Transactions of the Society of Electrochemical Science and Technology, 2006, 41: 94-96.
[15] Pruthviraj R D, Krupakara P V. A study on corrosion behaviour of ZA-27/Sic composite at higher temperature in acidic medium using autoclave [J]. Research Journal of Chemistry and Environment, 2006, 10: 71-75.
[16] Krupakara P V, MANJUNATH S, UCHIL J. Characterization of corrosion properties of ZA-27/quartz metal matrix composites [C]// Proceedings of Processing Materials for Properties 2000. San Francisco: TMS, 2000: 113-116.
[17] PacieJ R C, Aggarwala V S. Metallurgical variables influencing the corrosion susceptibility of a powder metallurgy SiCw/Al composite [J]. Corrosion, 1986, 42: 718-728.
[18] Sun H, Koo E Y, Wheat d H G. Corrosion behavior of SiCP/6061 Al metal matrix composites [J]. Corrosion, 1991, 47: 741-753.
[19] Trzaskoma P P. Pit morphology of aluminium alloy and silicon carbide/aluminium alloy metal matrix composites [J]. Corrosion, 1990, 46: 402-409.
[20] Osario W R, Freire C M, Garcia A. The effect of dendritic microstructure on the corrosion resistance of Zn-Al alloys [J]. Journal of Alloys and Compounds, 2005, 397: 179-191.
[21] Osario W R, Goulart P R, Santos G A, Netco C M, GARCIA A. Effect of dendritic arm spacing on mechanical properties and corrosion resistance of Al-9 wt pct Si and Zn-27 wt pct Al alloys [J]. Metallurgical and Materials Transactions A, 2006, 37A: 2525-2538.
[22] Prasad B K. Tensile properties of some zinc-based alloys comprising 27.5% Al: Effects of alloy composition, microstructure and test conditions [J]. Mater Sci Eng A, 1998, 245A: 257-266.
[23] Prasad B K. Response of some cast zinc-37.5%Al-based alloys comprising nickel/silicon under different tensile loading conditions [J]. Journal of Materials Engineering and Performance, 1998, 7: 632-636.
[24] Prasad B K. Mechanical properties and sliding wear characteristics of zinc-based alloys: Effects of partially substituting copper by silicon [J]. Zeitschrift fur Metallkunde, 1997, 88: 929-933.
[25] Prasad B K. Effects of silicon addition and test parameters on the sliding wear characteristics of zinc-based alloy containing 37.5% aluminium [J]. Materials Transactions of the Japan Institute of Metals, 1997, 38: 701-706.
[26] Savaskan T, Murphy S. Mechanical properties and lubricated wear of Zn-25 Al based alloys [J]. Wear, 1987, 116: 211-224.
[27] Murphy S, Savaskan T. Comparative wear behaviour of Zn-Al based alloys in an automotive engine application [J]. Wear, 1984, 98: 151-161.
[28] Prasad B K, Patwardhan A K, Yegneswaran A H. Microstructure-property characterization of some zinc-based alloys: Effects of heat treatment parameters [J]. Zeitschrift fur Metallkunde, 1996, 87: 967-971.
[29] Jian L, Laufer E E, Masounaive J. Wear in Zn-Al-Si alloys [J]. Wear, 1993, 165: 51-56.
[30] Prasad B K. Effect of heat treatment on tensile properties of zinc-37.5 mass % aluminium alloy containing nickel or silicon [J]. Materials Transactions of the Japan Institute of Metals, 1998, 39: 387-390.
[31] Lee P P, Savaskan T, Laufer E. Wear resistance and microstructure of Zn-Al-Si and Zn-Al-Cu alloys [J]. Wear, 1987, 117: 79-89.
[32] Prasad B K. Wear response of a zinc-based alloy containing silicon as influenced by material microstructure and test conditions [J]. Mater Sci Eng A, 2004, 367A: 63-73.
[33] Savaskan T, Adymer A. Effect of silicon content on the mechanical properties of monotectoid-based zinc-aluminium-silicon alloys [J]. Wear, 2004, 257: 377-388.
[34] Savaskan T, Bican o. Effect of silicon content on the microstructural features and mechanical and sliding wear properties of Zn-40Al-2Cu-(0-5)Si [J]. Mater Sci Eng A, 2005, 404A: 259-269.
[35] Prasad B K. Effects of partially substituting copper by silicon on the physical, mechanical and wear properties of a Zn-37.5%Al-based alloy [J]. Materials Characterization, 2000, 44: 301-308.
[36] Purcek G, Savaskan T, Murphy S. Dry sliding friction and wear properties of zinc-based alloys [J]. Wear, 2002, 252: 894-901.
[37] Savaskan T, turhal M s, Murphy S. Effects of cooling rate on structure and mechanical properties of monotectoid zinc-aluminium alloys [J]. Materials Science and Technology, 2003, 19: 67-74.
[38] AZAKLI Z, Savaskan T. An examination of friction and sliding wear properties of Zn-40Al-2Cu-2Si alloy in case of oil cut-off [J]. Tribology International, 2008, 41: 9-16.
[39] Savaskan T, AZAKLI Z. An investigation of lubricated friction and wear properties of Zn-40Al-2Cu-2Si alloy in comparison with SAE65 bearing bronze [J]. Wear, 2008, 264: 920-928.
[40] Savaskan T, kucomeroglu A P, Purcek G. Effects of copper content on the mechanical and sliding wear properties of monotectoid-based zinc-aluminium alloys [J]. Tribology International, 2004, 37: 45-50.
[41] Prasad B K, Patwardhan A K, Yegneswaran A H. Influence of heat treatment parameters on the microstructure and properties of some zinc-based alloys [J]. Journal of Materials Science, 1996, 31: 6317-6324.
[42] Prasad B K, Modi O P, Jha A K, Dasgupta R, Das S, Mondal D P, Yegneswaran A H. Erosion-corrosion-abrasion characteristics in slurry of a zinc-based alloy and Its composite containing alumina particle dispersoid [J]. Materials Science and Technology, 2001, 17: 1444-1450.
[43] Aylor D M, Moran P J. Effect of reinforcement on the pitting behaviour of Al-base MMCs [J]. Journal of the Electrochemical Society, 1985, 132: 1277-1281.
[44] Modi O P, Saxena M, Prasad B K, Yegneswaran A H, Vaidya M L. Corrosion behaviour of squeeze cast aluminium alloy-silicon carbide composites [J]. Journal of Materials Science, 1992, 27: 3897-3902.
[45] Saxena M, Modi O P, Prasad B K, Jha A K. Erosion-corrosion characteristics of an aluminium alloy-alumina fibre composite [J]. Wear, 1993, 169: 119-124.
[46] Turene S, Simard D, FisseT M. Influence of structural parameters on the slurry erosion resistance of squeeze cast metal matrix composite [J]. Wear, 1991, 149: 187-197.
[47] Turene S, Chatigny Y, Simard D, Varon S, Masounaive J. The effect of abrasive particles on the slurry erosion resistance of particulate reinforced aluminium alloy [J]. Wear, 1990, 141: 147-158.
[48] Levy A V. Erosion and erosion-corrosion of metals [J]. Corrosion, 1995, 51: 872-883.
[49] Mondal D P, Das S, Prasad B K. Study of erosive-corrosive wear behaviour of an aluminium alloy composite through factorial design of experiments [J]. Wear, 1998, 217: 1-6.
[50] Dasgupta R, Prasad B K, Jha A K, Modi O P, Das S, Yegneswaran A H. Slurry erosive wear characteristics of a hardfaced steel: Effect of experimental parameters [J]. Wear, 1997, 213: 41-46.
[51] Ramulu M, Raju S P, Inou H, Zeng J. Hydro abrasive-erosive characteristics of 30 vol.% SiC/6061-T6 Al composite at shallow impact angles [J]. Wear, 1993, 166: 55-63.
[52] Ji J L, Jang J L. Wear corrosion behaviour of cast-in composite reinforced by WC particles [J]. Wear, 1990, 138: 23-32.
[53] Li Y, Burstein G T, Hutchings I M. Influence of environmental composition and electrochemical potential on the slurry erosion-corrosion of aluminium [J]. Wear, 1995, 1818/183: 70-79.
[54] Tomilson W, Mathews S J. Cavitation erosion-corrosion of aluminium alloy matrix/ceramic composites [J]. Journal of Materials Science Letters, 1994, 13: 170-173.
[55] Das S, Mondal D P, Dasgupta R, Prasad B K. Mechanism of material removal during erosion-corrosion of an Al-SiC particle composite [J]. Wear, 1999, 236: 295-302.
[56] Yu S Y, Ishi H, Chuang T H. Corrosive wear of SiC whisker- and particulate reinforced 6061 aluminium alloy composites [J]. Metallurgical and Materials Transactions A, 1996, 27A: 2653-2662.
[57] ASTM Standards Designation G119-93. Standard Guide for Determining Synergism between Wear and Corrosion [R]. ASTM Standards, 1993: 139-144.
[58] Prasad B K. Effects of alumina dispersion on the erosive-corrosive wear response of a zinc-based alloy under changing slurry conditions and distance [J]. Wear, 2000, 238: 151-159.
[59] Prasad B K, Modi O P, Jha A K, Yegneswaran A H. Effect of sand concentration in the medium and traversal distance and speed on the slurry wear response of a zinc-based alloy alumina particle composite [J]. Tribology Letters, 2004, 17: 301-309.
[60] Prasad B K, Modi O P, Jha A K, Patwardhan A K. Effects of some material and experimental variables on the slurry wear characteristics of zinc-aluminium alloys [J]. Journal of Materials Engineering and Performance, 2001, 10: 75-80.
[61] Neville A, Hodgkiess T, Dallas J T. A study of the erosion–corrosion behavior of engineering steels for marine pumping applications [J]. Wear, 1995, 186/187: 497-507.
[62] Burstein G T, Li Y, Hutchings I M. The influence of corrosion on the erosion of aluminum by aqueous silica slurries [J]. Wear, 1995, 186/187: 515-522.
[63] Postlethwaite J. Effect of chromate inhibitor on the mechanical and electrochemical components of erosion-corrosion in aqueous slurries of sand [J]. Corrosion, 1981, 37: 1-5.
[64] Matsumura M, Oka Y, Hiura H, Yano M. The role of passivating film in preventing slurry erosion-corrosion of austenitic stainless steel [J]. ISIJ International, 1991, 31(2): 168-176.
[65] Neville A, REYS M, XU H. Examining corrosion effects and corrosion/erosion interactions on metallic materials in aqueous slurries [J]. Tribology International, 2002, 35: 643-650.
[66] Malka R, Nesic S, Gulino D A. Erosion-corrosion and synergistic effects in disturbed liquid particle flow [J]. Wear, 2007, 262: 791-799.
[67] Wu J K, Hsu Y S. Electrochemical corrosion of zinc in sodium chloride [J]. Journal of Materials Science, 1986, 21: 3475-3478.
[68] de la Fuente D, castario J G, Moroulo M. Long term atmospheric corrosion of zinc [J]. Corrosion Science, 2007, 49: 1420-1436.
[69] Farr J P G, Hampson N A. Reactions at solid metal electrodes Part 1—Faradaic impedance of zinc electrodes in alkaline solution [J]. Transactions of The Faraday Society, 1966, 62: 3493-3501.
[70] Farr J P G, Hampson N A. Evaluation of the characteristics of exchange reactions (1): Exchange reaction at a solid zinc electrode in alkali [J]. Transactions of Electroanalytical Chemistry, 1967, 13: 433-441.
[71] Van Dorne W, Dirkse T P. Supersaturated zincate solutions [J]. Journal of the Electrochemical Society, 1975, 122: 1-4.
[72] Ball A, Ward J J. An approach to material selection for corrosive-abrasive wear by systematic in-situ and laboratory testing procedures [J]. Tribology International, 1986, 85: 347-351.
[73] Zhou S, Stack M M, Newman R C. Characterization of synergistic effects between erosion and corrosion in an aqueous environment using electrochemical techniques [J]. Corrosion, 1996, 52: 934-946.
[74] Quintana P, Veleva L, Cauich W, Pomes R, Pene J L. Study of the composition and morphology of initial stages of corrosion products formed on Zn plates exposed to the atmosphere of southeast Mexico [J]. Applied Surface Science, 1996, 99: 325-334.
[75] Prasad B K, Patwardhan A K, Yegneswaran A H. Microstructural features controlling the sliding wear response of some bearing alloys [J]. Zeitschrift fur Metallkunde, 1997, 88: 774-780.
[76] PROTOPOPOFF E, MARCUS P. Electrode potentials [M]. Handbook of Metals: Corrosion-Fundamentals, Testing and Protection, Vol. 13A, 2003: 8-12, ASM, Materials Park, Ohio, USA.
[77] Brandes E A, ed. Smithells metals reference book [M]. 6th ed. London: Butterworths, 1983: 370-801.
[78] Prasad B K, Patwardhan A K, Yegneswaran A H. Dry sliding wear characteristics of some zinc-aluminium alloys: A comparative study with a bearing bronze at a slow speed [J]. Wear, 1996, 199: 142-151.
[79] Prasad B K, Patwardhan A K, Yegneswaran A H. Factors controlling the dry sliding wear behaviour of a leaded-tin bronze [J]. Materials Science and Technology, 1996, 12: 427-435.
Corresponding author: B. K. PRASAD; E-mail: braj-kprasad@yahoo.co.in
DOI: 10.1016/S1003-6326(08)60265-4
(Edited by YANG Hua)

