文章编号:1004-0609(2012)10-2938-06
Fe2+-Ni2+-NH3-NH4+-C2O42--H2O体系的
沉淀-配合平衡热力学
张传福,姚永林,湛 菁
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:针对草酸盐配位共沉淀-热分解还原法制备超细铁镍合金粉过程中Fe2+-Ni2+-NH3-NH4+-C2O42--H2O体系的溶液平衡建立热力学分析模型,并根据模型进行相关计算,揭示反应体系中各物质随pH值、氨及草酸浓度的变化关系。结果表明:溶液中的Fe主要以[Fe(C2O4)n]2-2n络合物形式存在,而铁氨络合物含量很低。当氨含量较低时,溶液中的Ni主要以[Ni(C2O4)n]2-2n存在;氨含量较高时,在酸性条件下,溶液中的Ni主要以[Ni(C2O4)n]2-2n存在,在碱性条件下,则主要以[Ni(NH3)n]2+存在。低pH值下,Ni的沉淀率较Fe的高,而高pH值下,Ni的沉淀率则较Fe的低。
关键词:铁镍合金;溶液平衡;络合;氨;草酸;pH值
中图分类号:TQ138.1 文献标志码:A
Thermodynamics of precipitation-coordination equilibrium in Fe2+-Ni2+-NH3-NH4+-C2O42--H2O system
ZHANG Chuan-fu, YAO Yong-lin, ZHAN Jing
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The thermodynamics model of the precipitation-coordination equilibrium in Fe2+-Ni2+-NH3-NH4+-C2O42--H2O system was established and the calculation of the model was carried out. The relationships of each substance in solution with pH value, ammonia and oxide acid were revealed. The results show that, Fe in solution exists as [Fe(C2O4)n]2-2n mainly in contrast to low content of [Fe(NH3)n]2+. At low ammonia content, Ni in solution exist as [Ni(C2O4)n]2-2n mainly. At high ammonia contents, Ni in solution exists as [Ni(C2O4)n]2-2n and [Ni(NH3)n]2+ under acidic and alkaline conditions, respectively. The precipitation rate of Ni is higher than that of Fe at low pH value, and lower than that of Fe at high pH value.
Key words: Fe-Ni alloy; solution equilibrium; coordination; ammonia; oxide acid; pH value
基金项目:高等学校博士学科点专项科研基金资助项目(20090162120080);湖南省科学技术厅科技计划一般项目(2010FJ3012)
收稿日期:2011-09-27;修订日期:2012-03-16
通信作者:张传福,教授,博士;电话:0731-88830471;E-mail: cfzhang2007@gmail.com
作为一种重要的过渡金属合金,铁镍合金由于具有较高磁导率和较低矫顽力等优异的磁电性能,而在电磁行业得到广泛应用[1]。超细铁镍合金粉因其尺寸小而均匀、比表面积大、化学活性高等特性以及特殊的表面磁性,在吸波材料[2-3]、磁性材料[4]、催化剂[5]、硬质合金[6]和合金镀层[7]等领域都有广泛的应用。目前制备超细铁镍合金粉的方法主要有机械合金法[8]、液相还原法[9-10]、沉淀法[11]、溶胶凝胶法[12]、微乳液 法[13]和电沉积法[14]等。沉淀法因工艺简单、过程易于控制、产品成分均匀等优点而成为液相制备粉体材料中最广泛采用的方法。在一般的沉淀过程中,沉淀剂和金属离子的混合容易导致局部浓度过大,大量晶核在此处产生,从而造成颗粒尺寸不均和严重的团聚现象。采用配位剂对金属离子预先进行络合,从而在沉淀过程中使其缓慢释放可以有效解决上述问题,以得到尺寸均匀的粒子[15]。
本课题组以氨为配位剂,通过草酸盐配位沉淀-热分解还原法已经成功地制备超细铁、镍、钴及镍钴合金粉[16-20],从而为采用该法制备超细铁镍合金粉提供了可能。但配位沉淀制备铁镍合金粉末前驱体的反应是一类极其复杂的体系,其中包括Fe2+、Ni2+与NH3、C2O42-形成配合物的反应,Fe2+、Ni2+与C2O42-生成草酸盐沉淀的反应,Fe2+、Ni2+的水解反应以及弱酸、弱碱的离解平衡反应等。本文作者针对Fe2+-Ni2+-NH3- NH4+-C2O42--H2O体系的离子平衡进行了计算,以确定共沉淀制备铁镍合金前驱体过程中pH值、氨及草酸浓度对沉淀过程的影响。
1 Fe2+-Ni2+-NH3-NH4+-C2O42--H2O系的离子平衡计算
在Fe2+-Ni2+-NH3-NH4+-C2O42--H2O反应体系中,NH3、C2O42-以及OH-均能与Fe2+、Ni2+发生一定程度的络合反应,各络合物的累积生成常数(β)如表1所 列[21]。
表1 络合物累积生成常数
Table 1 Cumulative formation constants of complexes

同时,溶液中存在草酸与氨的酸碱平衡反应,各反应平衡常数(K)如表2所列[21]。
表2 草酸与氨的酸碱平衡反应常数
Table 2 Equilibrium constants of oxalic acid and ammonia

[Fe]T、[Ni]T、[C2O4]T、[NH3]T及[OH]T为各物质在溶液中的分析浓度,综合表1、表2,根据质量平衡和同时平衡原理,可以得到如下各式。
[Fe]T=[Fe2+]+[FeC2O4]+[Fe(C2O4)22-]+[Fe(C2O4)34-]+[Fe(OH)+]+[Fe(OH)2]+[Fe(OH)3-]+[Fe(OH)42-]+[Fe(NH3)2+]+[Fe(NH3)22+] (1)
[Ni]T=[Ni2+]+[NiC2O4]+[Ni(C2O4)22-Ni(C2O4)34-]+[Ni(OH)+]+[Ni(OH)2]+[Ni(OH)3-]+[Ni(NH3)2+]+[Ni(NH3)22+]+[Ni(NH3)32+]+[Ni(NH3)42+]+[Ni(NH3)52+]+[Ni(NH3)62+] (2)
[C2O4]T=[C2O42-]+[HC2O4-]+[H2C2O4]+[FeC2O4]+2[Fe(C2O4)22-]+3[Fe(C2O4)34-NiC2O4]+2[Ni(C2O4)22-]+3[Ni(C2O4)34-] (3)
[NH3]T=[NH3]+[NH4+]+[Fe(NH3)2+]+2[Fe(NH3)22+]+[Ni(NH3)2+]+2[Ni(NH3)22+]+3[Ni(NH3)32+]+4[Ni(NH3)42+]+5[Ni(NH3)52+]+6[Ni(NH3)62+] (4)
[OH]T=[OH-Fe(OH)+]+2[Fe(OH)2]+3[Fe(OH)3-]+4[Fe(OH)42-Ni(OH)+]+2[Ni(OH)2]+3[Ni(OH)3-] (5)
在该反应体系中,一定条件下,Fe2+、Ni2+可能与C2O42-或OH-生成草酸盐沉淀或氢氧化物沉淀,各沉淀物的溶度积(Ksp)如表3所列[21]。
表3 Fe2+、Ni2+的草酸盐和氢氧化物沉淀的溶度积
Table 3 Solubility products of Fe2+, Ni2+ with C2O42- and OH-

当溶液中生成草酸盐沉淀时,金属离子浓度为
[Me2+]=Ksp/[C2O42-]
当pH上升到一定程度,草酸盐沉淀将会转变为氢氧化物沉淀,此时金属离子浓度为
[Me2+]=Ksp/[OH-]2=Ksp×1028-2pH
因此,在溶液中,Fe2+、Ni2+的浓度为
[Fe2+]= min{10-6.5/[C2O42-],1012.9-2pH}
[Ni2+]= min{10-9.4/[C2O42-],1013.3-2pH}
在一定草酸及氨浓度下,联立上述质量平衡方程、络合物生成方程、酸碱平衡方程以及沉淀平衡方程,采用MATLAB编程则可以求算出在不同条件下反应体系中各物质的浓度。
2 结果与讨论
2.1 [Fe]T与pH、[NH3]T及[C2O4]T的关系
图1所示为[C2O4]T=0.2 mol/L时溶液中[Fe]T与pH及[NH3]T的关系曲面。由图1可以看出,在pH<2时,由于FeC2O4沉淀在酸性条件下溶解,溶液中[Fe]T的含量较高。结合图2可知,此时Fe主要以游离Fe2+形式存在。随着pH升高,[Fe]T有所下降,这是因为此时H2C2O4和HC2O4-的离解平衡向右移动,造成C2O42-浓度增大,从而使FeC2O4沉淀率增大。pH继续增大,产生的C2O42-将和Fe2+大量络合,导致Fe2+沉淀率略微降低。pH在5~9之间时,酸碱平衡反应使[C2O42-]基本恒定,从而使其与Fe2+的络合物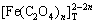 (Fe2+与C2O42-各络合物的总量)含量基本保持不变。结合图2中
(Fe2+与C2O42-各络合物的总量)含量基本保持不变。结合图2中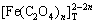 随pH的变化关系可知,在该pH范围内,[Fe(C2O4)n]2-2n是Fe存在的主要形式,因此,Fe2+的沉淀率基本保持不变。pH>9时,FeC2O4沉淀将向Fe(OH)2沉淀转变,导致[Fe]T急剧下降。但pH>12时,由于OH-与Fe2+的络合,Fe(OH)2沉淀将有一定程度的溶解,造成[Fe]T上升。由图2可知,在整个pH范围内,[NH3]T在0.2~1.0 mol/L变化时,铁氨络合物含量都远远低于Fe2+与C2O42-或OH-络合物的含量,因此,改变[NH3]T,Fe2+的沉淀率基本不变。
随pH的变化关系可知,在该pH范围内,[Fe(C2O4)n]2-2n是Fe存在的主要形式,因此,Fe2+的沉淀率基本保持不变。pH>9时,FeC2O4沉淀将向Fe(OH)2沉淀转变,导致[Fe]T急剧下降。但pH>12时,由于OH-与Fe2+的络合,Fe(OH)2沉淀将有一定程度的溶解,造成[Fe]T上升。由图2可知,在整个pH范围内,[NH3]T在0.2~1.0 mol/L变化时,铁氨络合物含量都远远低于Fe2+与C2O42-或OH-络合物的含量,因此,改变[NH3]T,Fe2+的沉淀率基本不变。

图1 [C2O4]T=0.2mol/L时[Fe]T与pH及[NH3]T的关系
Fig. 1 Relationships among [Fe]T, pH and [NH3]T at [C2O4]T= 0.2 mol/L
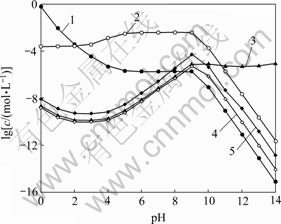
图2 [C2O4]T=0.2 mol/L时溶液中Fe的浓度与pH的关系
Fig. 2 Relationship of Fe concentration in solution and pH at [C2O4]T=0.2 mol/L: 1—[Fe2+]; 2—[Fe(C2O4)n]T2-2n; 3—[Fe(OH)n]T2-n; 4—[Fe(NH3)n]T2+, [NH3]T=0.2 mol/L; 5—[Fe(NH3)n]T2+, [NH3]T=1.0 mol/L
图3所示为[NH3]T=0.2 mol/L时[Fe]T与pH及[C2O4]T的关系曲面。由图3可知,pH<2时,随着[C2O4]T上升,[Fe]T有所下降。结合图4可知,在此pH范围内,改变[C2O4]T,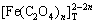 的含量基本没有变化,说明在较强的酸性条件下,C2O42-的沉淀作用起主导作用,从而引起Fe2+含量降低。当pH为3~11时,由图4可以看出,[Fe(C2O4)n]2-2n是Fe存在的主要形式,而随着[C2O4]T上升,
的含量基本没有变化,说明在较强的酸性条件下,C2O42-的沉淀作用起主导作用,从而引起Fe2+含量降低。当pH为3~11时,由图4可以看出,[Fe(C2O4)n]2-2n是Fe存在的主要形式,而随着[C2O4]T上升,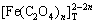 也相应增大,故图3中[Fe]T会随着[C2O4]T增大而上升。pH>11时,[Fe(OH)n]2-n是Fe存在的主要形式,此时[C2O4]T的变化对Fe2+沉淀率影响很小。
也相应增大,故图3中[Fe]T会随着[C2O4]T增大而上升。pH>11时,[Fe(OH)n]2-n是Fe存在的主要形式,此时[C2O4]T的变化对Fe2+沉淀率影响很小。
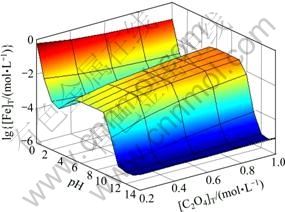
图3 [NH3]T=0.2 mol/L时[Fe]T与pH及[C2O4]T的关系
Fig. 3 Relationships among [Fe]T, pH and [C2O4]T at [NH3]T= 0.2 mol/L
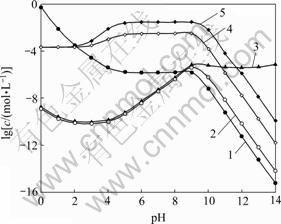
图4 [NH3]T=0.2 mol/L时溶液中Fe与pH的关系
Fig. 4 Relationship of Fe in solution and pH at [NH3]T= 0.2 mol/L: 1—[Fe2+]; 2—[Fe(NH3)n]T2+; 3—[Fe(OH)n]T2-n; 4— [Fe(C2O4)n]T2-2n, [C 2O4]T=0.2 mol/L; 5—[Fe(C2O4)n]T2-2n, [C2O4]T=1.0 mol/L
2.2 [Ni]T与pH、[NH3]T及[C2O4]T的关系
图5所示为[C2O4]T=0.2 mol/L时溶液中[Ni]T与pH及[NH3]T的关系曲面。由图5可以看出,在较强的酸性条件下,NiC2O4沉淀有一定程度的溶解,结合图6可知,此时溶液中存在主要存在着游离Ni2+。pH为1~12时,在[NH3]T较小的情况下,[Ni(C2O4)n]2-2n是Fe存在的主要形式,因此,[Ni]T随pH的变化规律与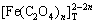 (Ni2+与C2O42-各络合物的总量)基本一致。但当[NH3]T增大时,在碱性条件下,溶液中NH3与Ni2+的络合作用将超过C2O42-与Ni2+的络合作 用,[Ni(NH3)n]2+是Ni存在的主要形式,因此图5中,pH>10时,[Ni]T会随着[NH3]T的增大而增大。由表1的氨络合物累积生成常数可知,造成[NH3]T对[Fe]T和
(Ni2+与C2O42-各络合物的总量)基本一致。但当[NH3]T增大时,在碱性条件下,溶液中NH3与Ni2+的络合作用将超过C2O42-与Ni2+的络合作 用,[Ni(NH3)n]2+是Ni存在的主要形式,因此图5中,pH>10时,[Ni]T会随着[NH3]T的增大而增大。由表1的氨络合物累积生成常数可知,造成[NH3]T对[Fe]T和
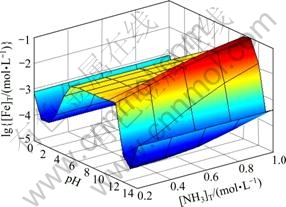
图5 [C2O4]T=0.2 mol/L时[Ni]T与pH及[NH3]T的关系
Fig. 5 Relationships among [Ni]T, pH and [NH3]T at [C2O4]T= 0.2 mol/L
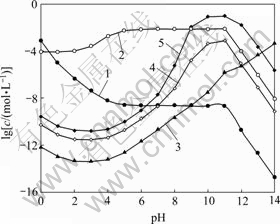
图6 [C2O4]T=0.2 mol/L时溶液中Ni与pH的关系
Fig. 6 Relationship of Ni in solution and pH at [C2O4]T=0.2 mol/L: 1—[Ni2+]; 2—[Ni(C2O4)n]T2-2n; 3—[Ni(OH)n]T2-n; 4—[Ni(NH3)n]T2+, [NH3]T=0.2 mol/L; 5—[Ni(NH3)n]T2+, [NH3]T=1.0 mol/L
[Ni]T作用不同的原因在于镍氨的络合能力远远大于铁氨的络合能力。
图7所示为[NH3]T=0.2 mol/L时 [Ni]T与pH及[C2O4]T的关系曲面。对比图3和图7,图4和图8,发现[Ni]T与[Fe]T随pH及[C2O4]T的变化趋势基本一致。只是在低pH条件下,[Ni]T较[Fe]T要低;而高pH条件下,[Ni]T则较高,其原因在于NiC2O4沉淀的溶度积比FeC2O4小而镍氨络合物累积生成常数则较高。
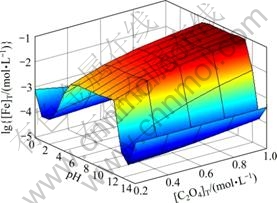
图7 [NH3]T=0.2 mol/L时[Ni]T与pH及[C2O4]T的关系
Fig. 7 Relationships among [Ni]T, pH and [C2O4]T at [NH3]T= 0.2 mol/L
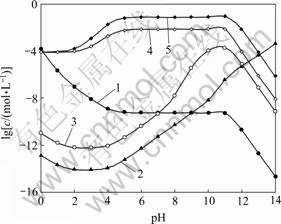
图8 [NH3]T=0.2 mol/L时溶液中Ni的浓度与pH的关系
Fig. 8 Relationships among Ni concentration in solution and pH at [NH3]T=0.2 mol/L: 1—[Ni2+]; 2—[Ni(OH)n]T2-n; 3—[Ni(NH3)n]T2+; 4—[Ni(C2O4)n]T2-2n, [C 2O4]T=0.2 mol/L; 5—[Ni(C2O4)n]T2-2n, [C2O4]T=1.0 mol/L
2.3 前驱体的成分控制
图9所示为当[C2O4]T=0.2 mol/L和[NH3]T=0.2 mol/L时溶液中[Fe]T和[Ni]T随pH的变化关系。由图9可以看出,当需要较高的金属离子沉淀率时,pH应

图9 [C2O4]T=0.2 mol/L和[NH3]T=0.2 mol/L时[Fe]T与[Ni]T和pH关系
Fig. 9 Relationships among [Fe]T, [Ni]T and pH at [C2O4]T= 0.2 mol/L and [NH3]T=0.2 mol/L
该控制在1~3之间。当前驱体需要维持溶液中铁镍的化学计量比时,pH应该控制在3.5(设铁镍的化学计量比为1:1)。当pH需要在一定区间内变化而又要保证前驱体中铁镍固定配比时,pH应该控制在5~9之间。总体来说,pH都应控制在9以下,因为pH>9时,将有Ni(OH)2沉淀生成,pH>11时,将有Fe(OH)2沉淀生成。
需要说明的是,Fe2+-Ni2+-NH3-NH4+-C2O42--H2O反应体系极其复杂,因而上述分析都是在一定条件下(如[C2O4]T=0.2 mol/L,[NH3]T=0.2 mol/L)所得的结果。而在其他条件下,各物质自身的性质保持不变,并都遵循质量平衡和同时平衡原理,因而各成分将呈现相似的变化趋势。在某一具体条件下,采用上述模型只需代入具体数值进行相应计算即可得出各物质的浓度分布。
3 结论
1) pH<2时,Fe主要以Fe2+形式存在;pH为2~11时,[Fe(C2O4)n]2-2n是Fe存在的主要形式,[Fe]T随着[C2O4]T的增大而增大;pH>11时,[Fe(OH)n]2-n是Fe存在的主要形式。整个pH范围内,[Fe(NH3)n]2+都很低,[NH3]T对[Fe]T基本没有影响。
2) 在[NH3]T较低的情况下,[Ni(C2O4)n]2-2n是Ni存在的主要形式,此时[Ni]T随[C2O4]T的增大而增大。[NH3]T较高时,随着pH增大,NH3与Ni2+的络合作用将超过C2O42-与Ni2+的络合作用,此时,[Ni(NH3)n]2+是Ni存在的主要形式。
3) pH<3.5时,Ni的沉淀率较Fe的高;pH>3.5时,Ni的沉淀率则比Fe的低。
REFERENCES
[1] DATTA A, PAL M, CHAKRAVORTY D, DAS D, CHINTALAPUDI S N. Disorder in nanocrystalline Ni3Fe[J]. Journal of Magnetism and Magnetic Materials, 1999, 205(2/3): 301-306.
[2] LI Xiang-cheng, GONG Rong-zhou, NIE Yan, ZHAO Zhen-sheng, HE Hua-hui. Electromagnetic properties of Fe55Ni45 fiber fabricated by magnetic-field-induced thermal decomposition[J]. Materials Chemistry and Physics, 2005, 94(2/3): 408-411.
[3] L? Rui-tao, KANG Fei-yu, CAI Dao-yan, WANG Chen, GU Jia-lin, WANG Kun-lin, WU De-hai. Long continuous FeNi nanowires inside carbon nanotubes: Synthesis, property and application[J]. Journal of Physics and Chemistry of Solids, 2008, 69(5/6): 1213-1217.
[4] 邹联隆, 易建宏, 李 强. 烧结工艺对注射成形Fe-50%Ni软磁材料磁性能的影响[J]. 粉末冶金材料科学与工程, 1999, 4(4): 310-315.
ZOU Lian-long, YI Jian-hong, LI Qiang. Effect of sintering process on magnetic properties in MIM Fe-50%Ni soft magnetic material[J]. Materials Science and Engineering of Powder Metallurgy, 1999, 4(4): 310-315.
[5] RODRIGUEZ N M, KIM M S, FORTIN F, MOCHIDA I, BAKER R T K. Carbon deposition on iron-nickel alloy particles[J]. Applied Catalysis A: General, 1997, 148(2): 265-282.
[6] 陈冰泉, 彭建中, 熊 英. A1-Si合金的氩弧表面铁、镍合金化研究[J]. 武汉理工大学学报: 交通科学与工程版, 2002, 26(1): 32-34.
CHEN Bing-quan, PENG Jian-zhong, XIONG Ying. A study of surface Fe-Ni alloying on argon arc of Al-Si alloy[J]. Journal of Wuhan University of Technology: Transportation Science and Engineering, 2002, 26(1): 32-34.
[7] 于金库, 王庚华, 邢广忠, 冯 皓. 在铜合金上获得FeNi合金镀层的电镀工艺研究[J]. 燕山大学学报, 1999, 23(2): 123-125.
YU Jin-ku, WANG Geng-hua, XING Guang-zhong, FENG Hao. A study of the plating processing about the coating of Ni-Fe alloy on copper alloy substrate[J]. Journal of Yanshan University, 1999, 23(2): 123-125.
[8] HAMZAOUI R, ELKEDIM O, GRENECHE J M, GAFFET E. X-ray diffraction and Mossbauer studies of mechanically alloyed Fe-Ni nanostructured powders[J]. Journal of Magnetism and Magnetic Materials, 2005, 294(2): 145-149.
[9] LIAO Qi-long, TANNENBAUM R, WANG Zhong-Lin. Synthesis of FeNi3 alloyed nanoparticles by hydrothermal reduction[J]. Journal of Physical Chemistry B, 2006, 110(29): 14262-14265.
[10] WEI Xian-wen, ZHU Guo-xing, ZHOU Ju-hong, SUN Hui-qun. Solution phase reduction to Fe-Ni alloy nanostructures with tunable shape and size[J]. Materials Chemistry and Physics, 2006, 100(2/3): 481-485.
[11] TANAKA A, YOON S H, MOCHIDA I. Formation of fine Fe-Ni particles for the non-supported catalytic synthesis of uniform carbon nanofibers[J]. Carbon, 2004, 42(7): 1291-1298.
[12] 沈宏芳, 陈文革, 谷臣清. 铁镍纳米粉末的制备及其表征[J]. 机械工程材料, 2005, 29(4): 31-33.
SHEN Hong-fang, CHEN Wen-ge, GU Chen-qing. Preparation and characterization of Fe-Ni nano-composite powders[J]. Materials for Mechanical Engineering, 2005, 29(4): 31-33.
[13] 胡 林, 张朝平. 镍/铁复合纳米微粒制备及颗粒尺寸的研究[J]. 复旦学报, 2003, 42(1): 35-38.
HU Lin, ZHANG Chao-ping. Preparation and characterization of Ni/Fe composite nanoparticles[J]. Journal of Fudan University, 2003, 42(1): 35-38.
[14] QIN X Y, LEE J S, NAM J G, KIM B S. Synthesis and microstructural characterization of nanostructured γ-Ni-Fe powder[J]. Nanostructured Materials, 1999, 11(3): 383-397.
[15] 黄 凯, 郭学益, 张多默. 超细粉末湿法制备工艺的粒子粒度和形貌控制[J]. 粉末冶金材料科学与工程, 2005, 10(5): 268-276.
HUANG Kai, GUO Xue-yi, ZHANG Duo-mo. Particle size and morphology control of ultrafine powders prepared by wet chemical precipitation[J]. Materials Science and Engineering of Powder Metallurgy, 2005, 10(5): 268-276.
[16] ZHANG Chuan-fu, ZHAN Jing, WU Jian-hui, LI Chang-jun. Preparation and characterization of fibrous NiO particles by thermal decomposition of nickelous complex precursors[J]. Transactions of Nonferrous Metals Society of China, 2004, 14(4): 713-717.
[17] ZHAN Jing, ZHANG Chuan-fu, LI Tie-jing, WU Jian-hui. Thermodynamic analysis on preparation of fibrous NiO precursor powders with oxalate precipitation process[J]. Transactions of Nonferrous Metals Society of China, 2005, 15(4): 926-930.
[18] ZHAN Jing, ZHOU Di-fei, ZHANG Chuan-fu. Shape-controlled synthesis of novel precursor for fibrous Ni-Co alloy powders[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 544-551.
[19] 湛 菁, 周涤非, 张传福, 贺跃辉, 岳建峰. 热分解含氨草酸钴复盐制备纤维状多孔钴粉[J]. 中南大学学报:自然科学版, 2011, 42(4): 876-883.
ZHAN Jing, ZHOU Di-fei, ZHANG Chuan-fu, HE Yue-hui, YUE Jian-feng. Preparation of fibrous porous cobalt powders by thermal decomposition of cobalt oxalate complex with ammonia[J]. Journal of Central South University: Science and Technology, 2011, 42(4): 876-883.
[20] 湛 菁, 邬建辉, 张传福, 岳建峰. 形貌控制合成纤维状氧化亚镍粉末新型前驱体[J]. 矿冶工程, 2010, 30(2): 64-69.
ZHAN Jing, WU Jian-hui, ZHANG Chuan-fu, YUE Jian-feng. Shape-controlled synthesis of novel precursor for preparing fiber-like nickel oxide powders[J]. Mining and Metallurgical Engineering, 2010, 30(2): 64-69.
[21] DEAN J A. 兰氏化学手册[M]. 北京: 科学出版社, 1991.
DEAN J A. Lange’s handbook of chemistry[M]. Beijing: Science Press, 1991.
(编辑 李艳红)