J. Cent. South Univ. (2012) 19: 319-323
DOI: 10.1007/s11771-012-1007-4
Preparation process and characterization of new Pt/stainless steel wire mesh catalyst designed for volatile organic compounds elimination
ZHANG Ting(张婷), CHEN Min(陈敏), GAO Yuan-yuan(高园园), ZHENG Xiao-ming(郑小明)
Institute of Catalysis, Zhejiang University, Hangzhou 310028, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: A novel 0.1% Pd-0.05% (mass fraction) Pt/stainless steel wire mesh catalyst was prepared for volatile organic compounds (VOCs) elimination. The catalyst was synthesized by stainless steel wire mesh as support and then treated by anodic oxidation technology to develop a porous membrane on the support. During the anodic oxidation process, various electrolytes were used to investigate the formation of porous membrane. And the catalytic performance of the catalysts was tested by using toluene and acetone combustion as model reaction. The temperatures of complete toluene and acetone conversion were decreased to 180 °C and 240 °C, respectively. The morphologies of the stainless steel wire mesh supports and catalysts were characterized by means of scanning electron microscopy (SEM) and temperature-programmed reduction (TPR).
Key words: volatile organic compounds; anodic oxidation; electrolyte; Pd
1 Introduction
Volatile organic compounds (VOCs) are the major components in air pollutants. Catalytic oxidation is one of the most effective methods for VOCs destruction [1-3]. Usually, cordierite honeycomb is used as typical catalyst support for catalytic combustion of VOCs [4]. But the shortcomings of low thermal stability and easy fragmentation for those catalysts using cordierite honeycomb as support have hindered their further development [5]. It is well known that metal materials such as stainless steel wire mesh [6-7] and aluminum template are very promising in aspects of high- thermal conductivity, low pressure drop and low manufacturing cost [8-9]. Nowadays, using metal substrates as catalyst supports has become more and more common [10-12]. One problem concerning using metal substrates as support is their low surface area, which restricts their application as catalyst support. In order to achieve larger surface area, many attempts have been tried, such as forming an inorganic oxide layer on the surface of metal support. Conventional washcoating methods are commonly used on ceramic cordierite honeycomb support monoliths [13]. However, they are not suitable for metallic monoliths because their slick surface results in poor adhesion and non-uniformity of coatings. Thus, developing an effective preparation technology for supporting active components on metal support is urgent. In Ref. [14], anodic oxidation technology was adopted in the treatment of stainless steel wire mesh support. This technology brought a layer of porous membrane on the stainless steel wire mesh and then active compounds such as Pd, Pt and Ce were well dispersed on the support.
Generally, the porous membrane layer grows on the metal surface using oxalic acid, sulphuric acid or phosphoric acid solutions as electrolytes during anodic oxidation process [15-17]. In order to further improve the catalytic performance of the catalysts, in this work, different kinds of electrolytes such as 5% acetic acid, 5% oxalic acid and 20% (mass fraction) sulphuric acid aqueous solution were used in anodic oxidation process during catalyst preparation. The influence of various electrolytes on catalysts for catalytic oxidation of toluene and acetone was evaluated.
2 Experimental
2.1 Preparation of catalysts
The stainless steel wire mesh (SSWM) was employed as the catalyst support. The SSWM was machined into a dimension of 400 mm×40 mm×0.3 mm with a rippled shape. After that, the SSWM was put into the acid solution in an isolated electrochemical cell and then anodic oxidation treatment was performed at room temperature. The anodic oxidation process was carried out under the following conditions: constant voltage of 3-5 V and electric current density of 1-2 A/cm2 at room temperature; 0.5 h of reaction time was performed to control the thickness of the anodic dielectric membrane; 5% acetic acid, 5% oxalic acid and 20% (mass fraction) sulphuric acid aqueous solution were used as electrolytes, respectively; the stirring rates were constant in the experiment all the time. Finally, anodic dielectric membrane appeared on the SSWM support surface.
After anodic oxidation treatment, the SSWM was washed in distilled water and dried at 100 °C for 1 h. Palladium and platinum catalysts were prepared by a wetness impregnation technique using the aqueous solution of H2PdCl4 and H2PtCl6 as precursor. The loading of Pd and Pt is 0.1% and 0.05% (mass fraction), respectively. The catalysts were subsequently washed by distilled water and then calcinated at 500 °C for 1 h in the air atmosphere. The catalysts are denoted as 0.1% Pd/SSWM and 0.1% Pd-0.05% Pt/SSWM, respectively (Table 1).
Table 1 Samples with various anodic oxidation electrolytes

2.2 Catalysts characterization
The morphologies of the SSWM support, 0.1% Pd/SSWM and 0.1% Pd-0.05% Pt/SSWM catalysts were characterized by scanning electron microscopy (SEM, JEM-T20). The temperature-programmed reduction (TPR) of the samples was carried out using H2/N2 (5%, volume fraction) as reducing agent at a flow rate of 25 cm3/min. The sample (0.35 g) was filled in a quartz tube, and treated in a flow of N2 for 20 min at room temperature. Then the sample was heated at a rate of 15 °C/min to 700 °C under the reducing mixture. Hydrogen consumption was monitored by a thermal conductivity detector (TCD).
2.3 Catalytic activity
The catalytic reactions of complete toluene and acetone oxidation over 0.1% Pd/SSWM and 0.1% Pd- 0.05% Pt/SSWM catalysts were carried out in a fixed bed reactor with length of 500 mm and inner diameter of 24 mm. The reactant flows were adjusted to the volume hour space velocity (VHSV) at 10 000 h-1 under atmospheric pressure and the reactant concentration was controlled in the range of 4-6 g/m3. The temperature was controlled by a programmed temperature controller. Both temperatures at the inlet and outlet of the SSWM catalysts were monitored. The outlet and inlet gas compositions were detected by a GC-8A gas chromatograph with a FID attachment. The reaction products were only CO2 and H2O, and no byproducts were found under the experimental conditions. The conversion was calculated based on toluene and acetone consumption.
3 Results and discussion
3.1 SEM results
Figure 1 shows the SEM images of SSWM supports with and without anodic oxidation technology. SSWM supports were treated by different acidic electrolytes at room temperature, including 5% acetic acid, 5% oxalic acid and 20% sulphuric acid aqueous solution during the anodic oxidation process (Figs. 1(b)-(d)). As seen in Fig. 1(a), the original SSWM support is smooth, on which it is hard to load active components. However, after anodic oxidation process, abnormal and multiangular structure layers appear on the surface of the SSWM. It is obvious that the presence of the porous membrane is beneficial to increasing the surface area of SSWM and favors the fixing of palladium and platinum active components.

Fig. 1 SEM images of SSWM supports with and without anodic oxidation technology with different electrolytes: (a) Un-treated SSWM support; (b) 5% acetic acid; (c) 5% oxalic acid; (d) 20% sulphuric acid
It is worthy mentioning that on the SSWM support treated by anodic oxidation electrolyte of 5% acetic acid, many pores appear on the membrane surface (Fig. 1(b)). However, the textures of the SSWM support treated by 20% sulphuric acid aqueous solution present few pores or cavities over the membrane surface. As for the SSWM supports treated by 5% oxalic acid, there is nearly no obvious change compared with Fig. 1(a). This may be related to the condition of anodic oxidation electrolyte in the catalyst preparation process. It is suggested that different kinds of electrolytes affect the membrane textures greatly.
Figure 2 displays the SEM images of 0.1% Pd/ SSWM-A and 0.1% Pd-0.05% Pt/SSWM catalysts, using 5% acetic acid as anodic oxidation electrolyte during catalyst preparation. It can be seen from Fig. 2(a) that the active components of Pd are well dispersed on the SSWM support and the particles are uniformly distributed over the membrane layer. This suggests a synergistic interaction between the support and active components due to the presence of the porous membrane. Figure 2(b) shows the SEM image of 0.1% Pd-0.05% Pt/SSWM catalyst. A more uniform distribution of Pd or Pt particles can be seen in comparison to the morphology of 0.1% Pd/SSWM-A catalyst, indicating that an interaction exists between palladium and platinum particles after addition of platinum [18-20].

Fig. 2 SEM images of catalysts: (a) 0.1% Pd/SSWM-A; (b) 0.1% Pd-0.05% Pt/SSWM
3.2. Activity measurement
The catalytic performance of mono-metallic and bi-metallic catalyst toward toluene and acetone oxidation is shown in Fig. 3. The catalytic reaction was kept at each temperature for about 0.5 h. The reactants of toluene and acetone were converted to CO2 and H2O completely. As can be seen from Fig. 3, among the mono-metallic catalysts, 0.1% Pd/SSWM-A shows the best catalytic activity. The temperatures of complete toluene and acetone conversion are 200 °C and 260 °C, respectively, while the other two catalysts exhibit lower catalytic activity. As for 0.1% Pd/SSWM-O, the temperature of complete toluene and acetone conversion is the highest. This is in good agreement with the SEM results. Thus, the good catalytic performance shown on 0.1% Pd/SSWM-A may be related to the appropriate condition of anodic oxidation electrolyte in the catalyst preparation process. It affects the morphology of the porous membrane, on which active components can be well dispersed and fastened on SSWM support, leading to the enhancement in toluene and acetone combustion.
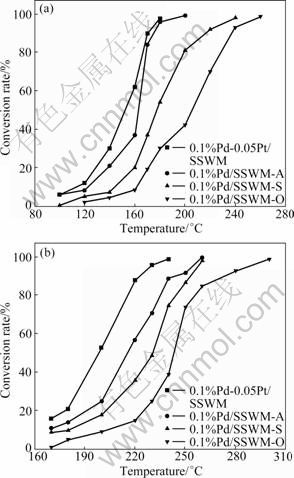
Fig. 3 Catalytic oxidation of toluene (a) and acetone (b) over different catalysts
Moreover, when 5% acetic acid was used as the anodic oxidation electrolyte, the 0.1% Pd-0.05% Pt/ SSWM catalyst exhibits better catalytic performance for toluene and acetone oxidation. The temperatures of complete toluene and acetone oxidation are declined to 180 °C and 240 °C, respectively, which is much lower than the temperatures reported in Refs. [21-22]. Compared with 0.1% Pd/SSWM-A catalyst, the addition of metal platinum brings an increase in the catalytic oxidation of toluene and acetone. Since 0.1% Pd-0.05% Pt/SSWM catalyst shows a good catalytic performance, it is suggested that there is a synergic interaction between the particles of Pd and Pt, which is responsible for the increased activity of toluene and acetone oxidation at low temperature, as SEKINE et al [23] reported.
3.3 H2-TPR results
H2-TPR profiles obtained over SSWM support, 0.1% Pd/SSWM-A and 0.1% Pd-0.05% Pt/SSWM are shown in Fig. 4. Figure 4(a) depicts the TPR results obtained from the blank samples of SSWM and it exhibits peaks at about 460 °C and 550 °C. These high temperature peaks can be associated with the reduction peak of FeOx and CrOx of SSWM support. In this work, all the catalysts are calcined at 500 °C and the reduction peaks which are assigned to SSWM support of these samples exhibit similar peaks at around 550 °C. As for 0.1% Pd/SSWM-A (Fig. 4(b)), a hydrogen-desorption peak at 80 °C is observed, suggesting a subsequent desorption of hydrogen from bulk palladium hydride [24]. In addition, less H2 is consumed in SSWM substrate area, reflected by the disappearance of the smaller peaks and shoulder, indicating that active components are in strong interaction with the porous membrane on SSWM support. In case of bi-metallic 0.1% Pd-0.05% Pt/SSWM catalyst (Fig. 4(c)), the reduction peak is slightly lower compared with the SSWM sample and a small reduction peak with a maximum reduction at 338 °C is observed. According to Ref. [25], the peak at 338 °C is attributed to the reduction of Pt species in strong interaction with the SSWM support. However, a hydrogen-desorption peak is not clearly observed, which might be caused by the interaction of the bi-metal and still needs more research. Moreover, the location of reduction peaks for the bimetallic Pd-Pt catalysts greatly depends on the degree of interaction between Pd and Pt. These results suggest that some sort of interaction between Pd and Pt particles and SSWM support occurs on 0.1% Pd-0.05% Pt/SSWM catalyst. The interaction may result in the high activity of 0.1%Pd-0.05%Pt/SSWM catalyst for the complete toluene and acetone oxidation.
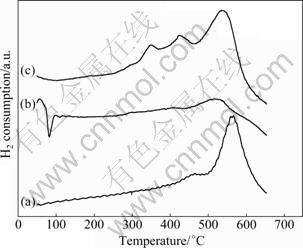
Fig. 4 H2-TPR profiles of samples over different catalysts: (a) SSWM; (b) 0.1% Pd/SSWM–A; (c) 0.1% Pd-0.05% Pt/ SSWM
3.4 Adherence test of porous membrane
In order to investigate the fastness of the porous membrane over SSWM support, the ultrasonic vibration test was carried out. As can be seen from Fig. 5, the mass loss-time data of the porous membrane prepared using 5% acetic acid as the anodic oxidation electrolyte over SSWM reveal some interesting facts. The mass loss of SSWM sample support is 0.23% after 10 min ultrasonic treatment. When the testing time is prolonged to 20 min, the mass loss is 0.25%, indicating that there are nearly no significant changes on the sample. Meanwhile, the mass loss data tend to hold a stable value when the test holds for 60 min. This suggests that the porous membrane on SSWM support shows a good adherence state even at a long time breakage test. This also indicates that a good synergistic interaction exists between the SSWM support and the porous membrane.
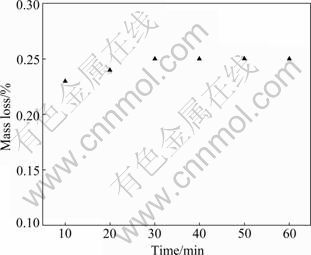
Fig. 5 Mass loss-time data of porous membrane on SSWM by ultrasonic vibration test
4 Conclusions
1) Various kinds of electrolytes are tried in the anodic oxidation process of catalyst preparation. 0.1% Pd-0.05% Pt/SSWM catalyst, using 5% acetic acid as the electrolyte in the anodic oxidation process, shows good catalytic performance for toluene and acetone oxidation. The temperatures of complete toluene and acetone conversion are 180 °C and 240 °C, respectively.
2) Anodic oxidation treatment over SSWM not only can overcome the drawbacks of low surface area of the SSWM support, but also favors the adhesion of the active components.
3) When low amount of noble metal is loaded, 0.1% Pd-0.05% Pt/SSWM exhibits optimal catalytic performance, which is owed to the synergistic interaction between the palladium, platinum and the anodic oxidation membrane on the SSWM support.
References
[1] AGUERO F N, BARBERO B P, GAMBARO L, CADUS L E. Catalytic combustion of volatile organic compounds in binary mixtures over MnOx/Al2O3 catalyst [J]. Applied Catalysis B: Environmental, 2009, 91(1/2): 108-112.
[2] SPINICCI R, FATICANTI M, MARINI P, ROSSI D, PORTA P. Catalytic activity of LaMnO3 and LaCoO3 perovskites towards VOCs combustion [J]. Journal of Molecular Catalysis A: Chemical, 2003, 197(1/2): 147-155.
[3] ZIMOWSKA M, MICHALIK-ZYM A, JANIK R, MACHEJ T, GURGUL J, SOCHA R P, PODOBINSKI J, SERWICKA E M. Catalytic combustion of toluene over mixed Cu-Mn oxides [J]. Catalysis Today, 2007, 119(1/2/3/4): 321-326.
[4] LUO Meng-fei, He Mai, XIE Yun-long, FANG Ping, JIN Ling-yun. Toluene oxidation on Pd catalysts supported by CeO2-Y2O3 washcoated cordierite honeycomb [J]. Applied Catalysis B: Environmental, 2007, 69(3/4): 213-218.
[5] SAHA B P, JOHNSON R, GANESH I, RAO G V N, BHATTACHARJEE S, MAHAJAN Y R. Thermal anisotropy in sintered cordierite monoliths [J]. Materials Chemistry and Physics, 2001, 67(1/2/3): 140-145.
[6] LOUIS B, REUSE P, KIWI-MINSKER L, RENKEN A. Synthesis of ZSM-5 coatings on stainless steel grids and their catalytic performance for partial oxidation of benzene by N2O [J]. Applied Catalysis A: General, 2001, 210(1/2): 103-109.
[7] LOUIS B, SUBRAHMANYAM C H, KIWI-MINSKER L, VISWANATHAN B, BUFFAT P A, RENKEN A. Synthesis and characterization of MCM-41 coatings on stainless steel grids [J]. Catalysis Communications, 2002, 3(4): 159-163.
[8] YANG K S, CHOI J S, CHUNG J S. Evaluation of wire-mesh honeycomb containing porous Al/Al2O3 layer for catalytic combustion of ethyl acetate in air [J]. Catalysis Today, 2004, 97(2/3): 159-165.
[9] SUNGKONO I E, KAMEYAMA H, KOYA T. Development of catalytic combustion technology of VOC materials by anodic oxidation catalyst [J]. Applied Surface Science, 1997, 121/122: 425-428.
[10] GAO L Z, KIWI-MINSKER L, RENKEN A. Growth of carbon nanotubes and microfibers over stainless steel mesh by cracking of methane [J]. Surface and Coatings Technology, 2008, 202(13): 3029-3042.
[11] SHAN Z, van KOOTEN W E J, OUDSHOORN O L, JANSEN J C, van BEKKUM H, van DEN BLEEK C M, CALIS H P A. Optimization of the preparation of binderless ZSM-5 coatings on stainless steel monoliths by in situ hydrothermal synthesis [J]. Microporous and Mesoporous Materials, 2000, 34(1): 81-91.
[12] VANDER WAL R L, HALL L J. Carbon nanotube synthesis upon stainless steel meshes [J]. Carbon, 2003, 41(4): 659-672.
[13] ZAMARO J M, ULLA M A, MIRO E E. Zeolite washcoating onto cordierite honeycomb reactors for environmental applications [J]. Chemical Engineering Journal, 2005, 106(1): 25-33.
[14] HAAK R P, SMITH T, INT J. Surface treatment of AM355 stainless steel for adhesive bonding [J]. International Journal of Adhesion and Adhesives, 1983, 3(1): 15-23.
[15] ZHOU Y K, SHEN C M, LI H L. Synthesis of high-ordered LiCoO2 nanowire arrays by AAO template [J]. Solid State Ionics, 2002, 146(1/2): 81-86.
[16] LI Yan, WANG Cheng-wei, ZHAO Li-rong, LIU Wei-min. Photoluminescence properties of porous anodic aluminium oxide membranes formed in mixture of sulfuric and oxalic acid [J]. Journal of Physics D: Applied Physics, 2009, 42: 1-5.
[17] SIGURDSON S, SUNDARAMURTHY V, DALAI A K, ADJAYE J. Effect of anodic alumina pore diameter variation on template-initiated synthesis of carbon nanotube catalyst supports [J]. Journal of Molecular Catalysis A: Chemical, 2009, 306(1/2): 23-32.
[18] NIQUILLE-ROTHLISBERGER A, PRINS R. Hydrodesulfurization of 4, 6-dimethyldibenzothiophene and dibenzothiophene over alumina-supported Pt, Pd, and Pt-Pd catalysts [J]. Journal of Catalysis, 2006, 242(1): 207-216.
[19] JIANG Heng, XU Yun, LIAO Shi-jian, YU Dao-rong, CHEN Hua, LI Xian-jun. A remarkable synergic effect of water-soluble bimetallic catalyst in the hydrogenation of aromatic nitrocompounds [J]. Journal of Molecular Catalysis A: Chemical, 1999, 142(2): 147-152.
[20] BALDOVINO-MEDRANO V G, ELOY P, GAIGNEAUX E M, GIRALDO S A, CENTENO A. Development of the HYD route of hydrodesulfurization of dibenzothiophenes over Pd–Pt/λ-Al2O3 catalysts [J]. Journal of Catalysis, 2009, 267(2): 129-139.
[21] RADIC N, GRBIC B, TERLECKI-BARICEVIC A. Kinetics of deep oxidation of n-hexane and toluene over Pt/Al2O3 catalysts Platinum crystallite size effect [J]. Applied Catalysis B: Environmental, 2004, 50(3): 153-159.
[22] GIL A, VICENTE M A, LAMBERT J F, GANDIA L M. Platinum catalysts supported on Al-pillared clay application to the catalytic combustion of acetone and methyl-ethyl-ketone [J]. Catalysis Today, 2001, 68(1/2/3): 41-51.
[23] SEKINE Y, TAKAMATSU H, ARAMAKI S, ICHISHIMA K, TAKADA M, MATSUKATA M, KIKUCHI E. Synergistic effect of Pt or Pd and perovskite oxide for water gas shift reaction [J]. Applied Catalysis A: General, 2009, 352(1/2): 214-222.
[24] CHOU C W, CHU S J, CHIANG H J, HUANG C Y, LEE C J, SHEEN S R, PERNG T P, YEH C T. Temperature-programmed reduction study on calcination of nano-palladium [J]. Journal of Physical Chemistry B, 2001, 105(38): 9113-9117.
[25] ZHANG Yi-wei, ZHOU Yu-ming, QIU An-ding, WANG Yu, XU Yi, WU Pei Cheng. Propane dehydrogenation on PtSn/ZSM-5 catalyst: Effect of tin as a promoter [J]. Catalysis Communications, 2006, 7(11): 860-866.
(Edited by HE Yun-bin)
Foundation item: Project(2009C21001) supported by the Science and Technology Program of Zhejiang Province, China
Received date: 2011-01-07; Accepted date: 2011-04-06
Corresponding author: CHEN Min, Professor, PhD; Tel: +86-13905710028; E-mail: chenmin@zju.edu.cn