Well-controlled stirring tank leaching to improve bio-oxidation efficiency of a high sulfur refractory gold concentrate
来源期刊:中南大学学报(英文版)2020年第5期
论文作者:尚鹤 武彪 温建康 刘美林 张其东 崔兴兰
文章页码:1416 - 1423
Key words:bio-oxidation; high-sulfur refractory gold concentrate; stirring leaching; pH control
Abstract: For the high sulfur refractory gold concentrate with 41.82% sulfur and 15.12 g/t gold, of which 82.11% was wrapped in sulfide, a well-controlled stirring tank leaching was carried out to improve the bio-oxidation efficiency. Results show that bio-oxidation pretreatment can greatly improve the gold recovery rate of high-sulfur refractory gold concentrate, and at the optimum pH 1.3 in this study, compared with the process without pH control, the oxidation rate of sulfur increased from 79.31% to 83.29%, while the recovery rate of gold increased from 76.54% to 83.23%; under this condition the activity of mixed culture could be sustained and the formation of jarosite could diminish. The results also displayed that for the high sulfur refractory gold concentrate, the recovery of gold is positively correlated with the oxidation rate of sulfur, and the recovery rate of gold increases with the increase of sulfur oxidation rate within a certain range.
Cite this article as: WU Biao, SHANG He, WEN Jian-kang, LIU Mei-lin, ZHANG Qi-dong, CUI Xing-lan. Well-controlled stirring tank leaching to improve bio-oxidation efficiency of a high sulfur refractory gold concentrate [J]. Journal of Central South University, 2020, 27(5): 1416-1423. DOI: https://doi.org/10.1007/s11771-020-4377-z.

J. Cent. South Univ. (2020) 27: 1416-1423
DOI: https://doi.org/10.1007/s11771-020-4377-z
WU Biao(武彪)1, 2, 3, 4, SHANG He(尚鹤)1, 2, 3, 4, WEN Jian-kang(温建康)1, 2, 4,
LIU Mei-lin(刘美林)1, 2, 4, ZHANG Qi-dong(张其东)1, 2, CUI Xing-lan(崔兴兰)1, 2
1. National Engineering Laboratory of Biohydrometallurgy, GRINM Group Co., Ltd., Beijing 100088, China;
2. GRINM Resources and Environment Tech. Co., Ltd., Beijing 101407, China;
3. GRIMAT Engineering Institute Co., Ltd, Beijing 101407, China;
4. General Research Institute for Nonferrous Metals, Beijing 100088, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: For the high sulfur refractory gold concentrate with 41.82% sulfur and 15.12 g/t gold, of which 82.11% was wrapped in sulfide, a well-controlled stirring tank leaching was carried out to improve the bio-oxidation efficiency. Results show that bio-oxidation pretreatment can greatly improve the gold recovery rate of high-sulfur refractory gold concentrate, and at the optimum pH 1.3 in this study, compared with the process without pH control, the oxidation rate of sulfur increased from 79.31% to 83.29%, while the recovery rate of gold increased from 76.54% to 83.23%; under this condition the activity of mixed culture could be sustained and the formation of jarosite could diminish. The results also displayed that for the high sulfur refractory gold concentrate, the recovery of gold is positively correlated with the oxidation rate of sulfur, and the recovery rate of gold increases with the increase of sulfur oxidation rate within a certain range.
Key words: bio-oxidation; high-sulfur refractory gold concentrate; stirring leaching; pH control
Cite this article as: WU Biao, SHANG He, WEN Jian-kang, LIU Mei-lin, ZHANG Qi-dong, CUI Xing-lan. Well-controlled stirring tank leaching to improve bio-oxidation efficiency of a high sulfur refractory gold concentrate [J]. Journal of Central South University, 2020, 27(5): 1416-1423. DOI: https://doi.org/10.1007/s11771-020-4377-z.
1 Introduction
Gold ores are mainly categorized into two types: non-refractory (fine milling) gold ores, in which most of the gold can be recovered by direct cyanidation, and refractory gold ores, which require pretreatment before cyanidation [1, 2]. As gold resources continue to be mined, mines that are easy to treat are decreasing, and re-factory gold mines will become the main resources of the gold industry in the future [3-6]. According to statistics, about one third of the world’s total gold production is from refractory gold mines, and this proportion will definitely increase in the future [7, 8]. Generally, the formation of refractory sulfide gold deposits is closely related to sulfide minerals, such as pyrite, arsenopyrite, pyrrhotite and chalcopyrite, among which pyrite is the most important gold-bearing mineral [9]. Gold is usually embedded in the crystal lattice of the pyrite as extremely fine particles or submicroscopic morphology, and sometimes even distributed in the pyrite as disseminated particles. It is impossible to dissociate the monomer by fine grinding and ultra-fine grinding and difficult to extract gold by the traditional cyanide leaching method. In order to achieve the economic and feasible recovery rate of gold, it is necessary to carry out oxidation pretreatment before the gold leaching process [10, 11], to open the package of gold and eliminate the influence of harmful impurities on the subsequent gold extraction [12, 13].
Pretreatment methods include roasting, pressure oxidation, chemical oxidation or fine grinding, to render the gold amenable to extracting [14-16]. There is also bio-oxidation for refractory ores, which offers a great advantage compared to the other pretreatment methods, as reagent costs are low and the process is carried out under mild conditions, which reduces the operational cost and environmental impact [17-21].
In recent years, biological pre-oxidation has attracted more and more attention. However, there is one obvious disadvantage of this method, which is the long leaching cycle. One of the important reasons for these problems is that the accumulation of acids in the pre-oxidation process affects the activity of bacteria, and the passivation on the surface of sulfide minerals in the leaching process directly affects the interaction between bacteria and minerals [22], leading to a decline in the leaching rate [17]. In order to intensify the bio-oxidation process, chemical-biological two-stage pre- oxidation approaches were carried out by some scholars [17, 23]. On the other hand, some have carried out the research of subsection biological oxidation [24].
To improve bio-oxidation efficiency of a high sulfur refractory gold concentrate, a well-controlled stirring preoxidation leaching experiment was carried out. This work will study the factor couplings during stirring tank bio-oxidation tests, and the detailed factors studied are as follows: pH of bio-oxidation solution and temperature.
2 Materials and methods
2.1 Stirring tank bio-oxidation system
Figure 1 shows the structure of stirring tank in bio-oxidation system, which can deal with 3 L pulp, meeting the requirement of this work.
Stirring tank and automatic potentiometric titrator can be seen in the figure as the two main components of the system. Double-layered glass mixing tank is with thermostat water circulation in the jacket. The system can accurately control the mixing speed, pulp pH value and pulp temperature. The automatic potentiometric titrator can measure the pH value of pulp online and automatically add potassium hydroxide solution (1 mol/L) to maintain the pH value of pulp at a specific value. The constant temperature water bath can control the temperature of pulp [25]. Figure 2 shows the actual structure of the test equipment used in this work.
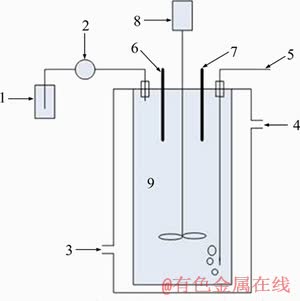
Figure 1 Diagrammatic drawing of stirring tank bio-oxidation system: 1-Potassium hydroxide solution (1 mol/L); 2-Automatic potentiometric titrator (Metrohm 902 Titrando); 3-Water inlet connected to constant temperature tank; 4-Water outlet returning water back to constant temperature tank; 5-Inflatable tube; 6-pH electrode; 7-Thermometer; 8-Agitator; 9-Stirring tank

Figure 2 Photo of stirring tank bio-oxidation system
2.2 Characterization of mineral samples
The original sample was obtained from Guizhou Province of China, and high-sulfur gold concentrate was obtained after crushing, grinding and flotation. The concentrate sample was finely ground, and the particle size of the sample was 90% smaller than 0.037 mm. The processed sample was utilized in the chemical analysis and the chemical element distribution of the high-sulfur gold concentrate sample is listed in Table 1 and Table 2 lists the gold phases of the sample. According to the sample analysis results, the gold grade was 15.12 g/t, and the sample was mainly composed of sulfide ore, with sulfur content of 41.82% and iron content of 38.82%. XRD patterns (Figure 3) showed that the concentrate contained pyrite as the major sulfide phase and phase results displayed 82.11% of the gold in sulfide ore.
Table 1 Chemical elements distribution of mineral samples
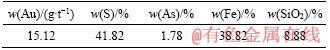
Table 2 Gold phases of mineral samples

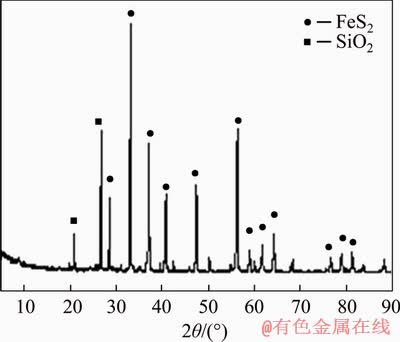
Figure 3 XRD pattern of mineral sample
2.3 Microorganisms and culture medium
Mixed culture used in this study was composed of Sulfobacillus thermotolerans, Leptospirillum ferriphilum and Ferroplasma acidiphilum, saved in National Engineering Laboratory of Biohydrometallurgy (GRINM Group Corporation Limited) [26]. The mixed culture was cultured in 100 mL of 9K basal medium [27, 28] in 250-mL Erlenmeyer flask at 45 °C on a rotary shaker (150 r/min). The solution pH of enrichment medium was adjusted to 1.5 with dilute sulphuric acid [23]. The compositions of 9K medium were 3 g/L (NH4)2SO4, 0.5 g/L K2HPO4, 0.5 g/L MgSO4·7H2O, 0.1 g/L KCl, 0.01 g/L CaNO3 and 44.6 g/L FeSO4·7H2O.
2.4 Bio-oxidation of concentrates
The research of the bio-oxidation of the high-sulfur gold concentrate was carried out using two series of experiments: (a) a process without pH control (initial pH 1.5) but with temperature control (45 °C) in the whole process; (b) a process in which initial pH was 1.5 and the pre-oxidation pH was controlled to be not less than 1.5, 1.3, 1.0, 0.7, respectively, and temperature was controlled at 45 °C in the whole process. The experiments were conducted in 3-L stirring tank that contained 2 L pulp, which was prepared from tap water and gold concentrate without adding medium and with the pulp density 15%. After the strain was cultured, it was inoculated into the pulp, and the inoculum of the strain was 10% (V/V). The solution pH was adjusted online in Group (b). When the pH is lower than the set value, the system would automatically add potassium hydroxide solution to keep the pH of the slurry at the set value. The test time was 3, 6, 9, 12 and 15 d. The experiments were conducted with a stirring speed of 300 r/min and an aeration rate of 5 L/min. The contents of sulfur and arsenic in pre-oxidized slag were determined after the test.
2.5 Cyanidation tests
The products of sulfide bio-oxidation were filtered and washed several times with de-ionized water. Samples were leached at a pulp density of 20% (V/W) for 24 h at pH 11. The pH was adjusted using CaO and cyanide strength was 10 kg/t. The remaining gold in the residue was determined by conventional fire assay method.
2.6 Analysis methods
The pH values of the leaching solution were determined with Metrohm 902 Titrando and the potential measurement was conducted with SUNTEX PC-350. At the end of the cyanide leaching tests, the concentration of gold was determined by inductively couple plasma/optical emission spectrometry (ICP-OES).
3 Results and discussion
3.1 Bio-oxidation of concentrates without pH control
In the process of biological pre-oxidation, as the pyrite and other sulfide minerals are rapidly oxidized, the acid in the system is continuously accumulated, causing the pH value of the system to continuously decrease, affecting the activity of microorganisms. Figure 4 shows that after inoculation, the pH value of the system decreased from 1.5 to about 0.6 with the progress of pre- oxidation, while the system redox potential increased to 690 mV from 350 mV. Meanwhile, we can see that the pH value of the system decreased rapidly and the redox potential increases rapidly after the inoculation of bacteria for 3 d. After 12 d of bio-oxidation, the pH value of the system decreased to 0.71, after which the decline trend of the pH value of the system slowed down. Without adjusting pH value, sulfur oxidation rate reached 79.31% and arsenic oxidation rate reached 76.92% after 15 d of oxidation pretreatment (Table 3). In addition, it also can be seen from Figure 5 that compared with the gold concentrate, the main body of the gold-bearing mineral in the oxidized slag has been destroyed by microbial oxidation, and the crack was irregularly penetrated throughout the gold concentrate particles. And under this condition, the surface of the gold concentrate did not form a passivation layer after pre-oxidation.
3.2 Bio-oxidation of concentrates with pH control
In order to prevent microbial activity from being affected by the continuous accumulation of acids in the system during the pre-oxidation process, online pH monitoring and real-time pH adjustment were adopted in this study. As can be seen from Figure 6, when the pH value of the system remains at 1.5 and 1.3, the redox potential of the system does not rise to more than 690 mV as shown in Figure 4, but fluctuates between 440 and 480 mV until the end of pre-oxidation. When the pH value of the system was controlled at 1.0, the redox potential of the system can rise to about 550 mV in the later stage of biological pre-oxidation, and to 650 mV when the pH value was controlled at 1.0.
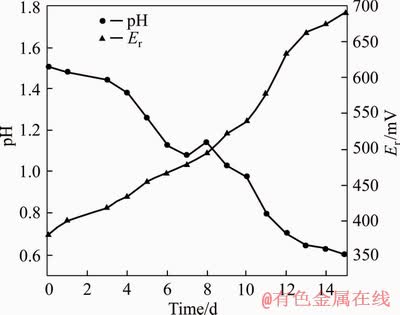
Figure 4 pH and redox potential Er of system
Table 3 Sulfur and arsenic oxidation rate
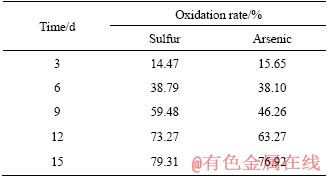
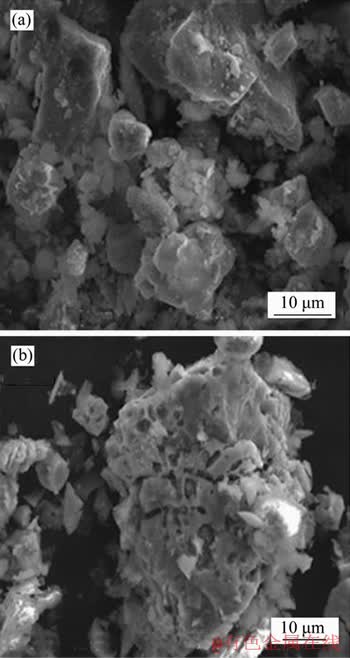
Figure 5 SEM images of gold concentrate (a) and oxidized residues (b)
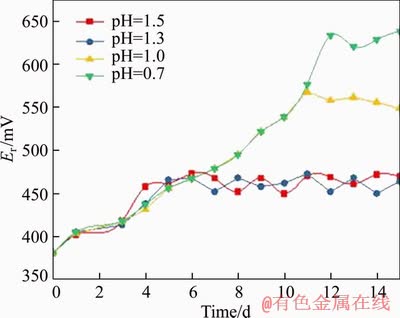
Figure 6 Redox potential of system
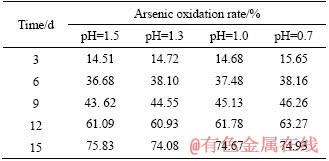
The effects of pH control on sulfur oxidation are listed in Table 4. In the process of biological oxidation, the oxidation rate of sulfur decreases with the decline of pH value of the system. In the range of pH value 1.3-1.5, sulfur oxidation is basically unaffected; after 15 d of biological pre-oxidation, the oxidation rate of sulfur can reach 80% or more. When the pH value was lower than 1.3, the oxidation levels were influenced accordingly; when the pre-oxidation cycle was 15 d, and the oxidation rate of sulfur was reduced from 83.29% to less than 75.25%. At the same time, during the pre-oxidation process, when the pH value of the system was above 1.3, a large amount of jarosite precipitate was formed on the surface of the concentrate, forming a dense passivation layer, which deposited in the form of grape spheres on the surface of the concentrate (Figure 7). Obviously, the pH of the solution should be maintained at about 1.3 in the biological pre-oxidation. Under such conditions, the activity of the microorganism is not affected, and the high oxidation efficiency can be maintained, while the jarosite precipitate is rarely produced. During the test, we found that the oxidation of arsenic was basically unaffected when controlling pH.
Table 4 Sulfur oxidation rate
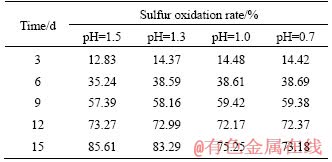
Table 5 Arsenic oxidation rate
3.3 Cyanidation tests
The concentrates and oxidized slag under various conditions were subjected to the cyanide leaching tests to determine the oxidation efficiency. Obviously, the recovery rate of gold from the original concentrates sample was only15.72%; after 15 d of biological pre-oxidation, the recovery rate of gold can be greatly increased to more than 80%. Table 6 shows that controlling pH value during the pre-oxidation process, especially when the pH value was 1.3, could effectively improve the recovery of gold. In this case the gold recovery rate increased to 83.23%, 6.69% higher than the method without pH control. When the pH value was higher than 1.3, a large amount of jarosite precipitation would be generated, which would affect the recovery of gold; when the pH value was lower than 1.3, it would affect the activity of bacteria, thereby affecting the oxidation rate of sulfur and recovery of gold. Meanwhile, it could be found from Table 6 that, for the high sulfur refractory gold concentrate, the recovery of gold was positively correlated with the oxidation rate of sulfur, and the recovery rate of gold increases with the increase of sulfur oxidation rate. However, when the sulfur oxidation rate reached 50% or more, the recovery rate of gold decreased.
4 Conclusions
1) Bio-oxidation pretreatment can greatly improve the gold recovery rate of high-sulfur refractory gold concentrate. After 15 d of bio- oxidation, whether the pH of the solution was controlled, the gold recovery rate could be increased from 15.72% to more than 75.11%.
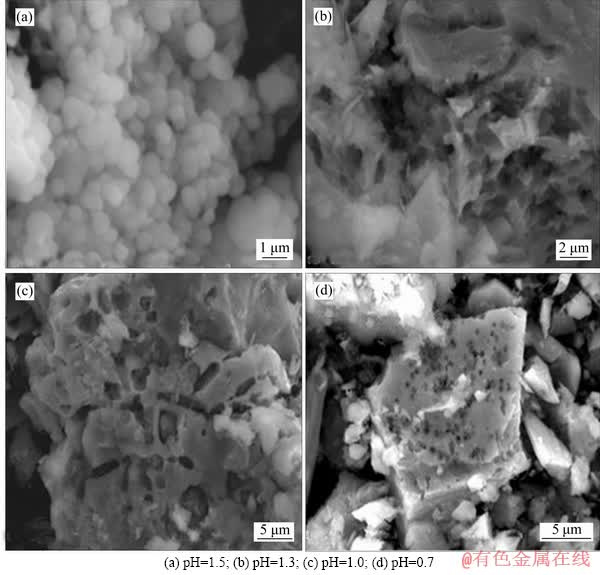
Figure 7 SEM images of oxidizing residues:
Table 6 Gold recovery at different oxidation stages
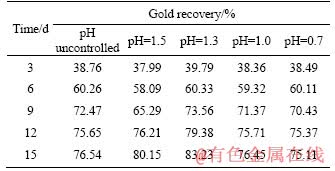
2) Due to the presence of Ferroplasma acidiphilum, the biological pre-oxidation process could be carried out in a lower pH range. The optimum pH was 1.3 in this study and compared with the process without pH control, the oxidation rate of sulfur increased from 79.31% to 83.29%, while the recovery rate of gold increased from 76.54% to 83.23%. Controlling the pH in a certain range during the bio-oxidation process can effectively increase the oxidation rate of sulfur.
3) For the high sulfur refractory gold concentrate, the recovery of gold was positively correlated with the oxidation rate of sulfur, and the recovery rate of gold increased with the increase of sulfur oxidation rate. However, when the sulfur oxidation rate reached 50% or more, the gold leaching rate decreased slowly.
References
[1] QIU Xiao-bin, WEN Jian-kang, HUANG Song-tao, YANG Hong-ying, LIU Mei-lin, WU Biao. New insights into the extraction of invisible gold in a low-grade high-sulfur Carlin-type gold concentrate by bio-pretreatment [J]. International Journal of Minerals Metallurgy and Materials, 2017, 24(10): 1104-1111. DOI:10.1007/s12613-017-1501-7.
[2] AFENYA P M. Treatment of carbonaceous refractory gold ores [J]. Minerals Engineering, 1991, 4: 7-11. DOI: 10.1016/ 0892-6875(91)90082-7.
[3] FRASER K S, WALTON R H, WELLS J A. Processing of refractory gold ores [J]. Minerals Engineering, 1991, 4(7-11): 1029-1041. DOI: 10.1016/0892-6875(91)90081-6.
[4] JIANG Tao, LI Qian, YANG Yong-bin, LI Guang-hui, QIU Guan-zhou. Bio-oxidation of arsenopyrite [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1433-1438. DOI: 10.1016/s1003-6326(09)60021-1.
[5] LA BROOY S R, LINGE H G, WALKER G S. Review of gold extraction from ores [J]. Minerals Engineering, 1994, 7(10): 1213-1241. DOI: 10.1016/0892-6875(94)90114-7.
[6] GONZALEZ R, GENTINA J C, ACEVEDO F. Continuous biooxidation of a refractory gold concentrate [J]. Process Metallulgy, 1999(9): 309-317. DOI: 10.1016/S1572-4409 (99)80031-5.
[7] WANG K L, WANG M H, JIANG J L. Current status of studies on bacterial oxidation and pre-disposal of refractory gold ores [J]. Gold Science and Technology, 2001, 9(1): 19-25.
[8] RAMON G, JUAN C, FEKNANDO A.Biooxidation of a gold concentratein a continuous stirred tank reactor: mathematical model and optimal configuration [J]. Biochemical Engineering Journal, 2004(19): 33-42. DOI: 10.1016/j.bej.2003.09.007.
[9] NATALYA V, FOMCHENKO, TAMARA F, KONDRAT’EVA, MAXIM I, MURAVYOV. A new concept of the biohydrometallurgical technology for gold recovery from refractory sulfide concentrates [J]. Hydrometallurgy, 2016, 164: 78-82. DOI: 10.1016/j.hydromet.2016.05.011.
[10] MUBAROK M Z, WINARKO R, CHAERUN S K, RIZKI I N, ICHLAS Z T. Improving gold recovery from refractory gold ores through biooxidation using iron-sulfur-oxidizing/ sulfur-oxidizing mixotrophic bacteria [J]. Hydrometallurgy, 2017, 168: 69-75. DOI: 10.1016/j.hydromet.2016.10.018.
[11] CHEN Bo-wei, SUN Jian-zhi, SHANG He, WU Biao, WEN Jian-kang. Biooxidation of a refractory gold ore: Implications of whole-ore heap biooxidation [J]. Solid State Phenomena, 2017, 262: 65-69, DOI: 10.4028/www.scientific. net/SSP.262.65.
[12] FRASER K S, WALTON R H, WELLS J A. Processing of refractory gold ore [J]. Minerals Engineering, 1991, 4(7-11): 1029-1041. DOI: 10.1016/0892-6875(91)90081-6.
[13] YANG L L, YANG H Y, FAN Y J, WANG D W, ZHU C L, SUN H L. Study on influencing factors of bacterial oxidation of refractory gold ores [J]. Precious Metal, 2007, 28(1): 58-62. DOI: 10.1016/s1872-2067(07)60020-5.
[14] CLIMO M, WATLING H R, van BRONSWIJK W. Biooxidation as pre-treatment for a telluride-rich refractory gold concentrate [J]. Minerals Engineering, 2000, 13: 1219-1229. DOI: 10.1016/S0892-6875(00)00106-0.
[15] HENLEY K J. Gold ore mineralogy and its relation to metallurgical treatment, [J]. Minerals Science and Engineering, 1975(7): 289-312. DOI: https://doi.org/10. 1007/978-1-4684-8425-0_5.
[16] GAO G, LI D, ZHOU Y, SUN X, SUN W. Kinetics of high-sulphur and high-arsenic refractory gold concentrate oxidation by dilute nitric acid under mild conditions [J]. Miner Eng, 2009, 22(2): 111-115. DOI: 10.1016/j.mineng. 2008.05.001.
[17] WANG Guo-hua, XIE Shui-bo, LIU Xin-xing, WU Yong-hong, LIU Ying-jiu, ZENG Tao-tao. Bio-oxidation of a high-sulfur and high-arsenic refractory gold concentrate using a two-stage process [J]. Minerals Engineering, 2018, 120: 94-101. DOI: https://doi.org/10.1016/j.mineng.2018. 02.013.
[18] BRIERLEY J A, KULPA C F. Microbial consortium treatment of refractory precious metal ores [P]. US Patent, 1992.
[19] IGLESIAS N, CARRANZA F. Refractory gold-bearing ores: A review of treatment methods and recent advances in biotechnological techniques [J]. Hydrometallurgy, 1994, 34(3): 383-395. DOI: 10.1016/0304-386X(94)90074-4.
[20] CLIMO M, WATLING H R, BRONSWIJK V W. Biooxidation as pre-treatment for a telluride-rich refractory gold concentrate [J]. Minerals Engineering, 2000, 13(12): 1219-1229. DOI: 10.1016/S0892-6875(00)00106-0.
[21] NIEVES I, FRANCISCO C. Refractory gold-bearing ores: A review of treatment methods and recent advances in biotechnological techniques [J]. Hydrometallurgy, 1994, 34(3): 383-395. DOI: 10.1016/0304-386X(94)90074-4.
[22] FENG Ya-li, WANG Hong-jun, LI Hao-ran, CHEN Xi-pei, DU Zhu-wei, KANG Jin-xing. Effect of iron transformation on Acidithiobacillus ferrooxidans bio-leaching of clay vanadium residue [J]. Journal of Central South University, 2019, 26(4): 796-805. DOI: https://doi.org/10.1007/s11771- 019-4049-z.
[23] LIU Xin-xing, WANG Guo-hua, HUO Qiang, XIE Jian-ping, LI Shou-peng, WU Hai-yan, GUO Yu-jie. Novel two-step process to improve efficiency of bio-oxidation of Axi high-sulfur refractory gold concentrates [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4119-4125. DOI: 10.1016/S1003-6326(15)64063-8.
[24] QIU Xiao-bin, WEN Jian-kang, WU Biao, ZOU Lai-chang, LIU Mei-lin, SHANG He. Biooxidation pretreatment of high sulfur high clay carlin-type gold concentrates containing arsenic and carbon [J]. Chinese Journal of Rare Metals, 2013, 37(5): 783-790. DOI: 10.3969/j.issn.0258-7076.2013.05. 017. (in Chinese)
[25] WANG Jun, HU Ming-hao, ZHAO Hong-bo, TAO Lang, GAN Xiao-wen, QIN Wen-qing, QIU Guan-zhou. Well- controlled column bioleaching of a low-grade copper ore by a novel equipment [J]. Journal of Central South University, 2015, 22(9): 3318-3325. DOI: 10.1007/s11771-015-2872-4.
[26] HE Shang, WEN Jian-kang, WU Biao. Bio-pretreatment and community analysis for high sulfur arsenic-bearing refractory gold concentrate [J]. Chinese Journal of Rare Metals, 2013, 37(6): 976-983. DOI: 10.3969j.issn.0258- 7076.2013.06019. (in Chinese)
[27] SILVERMAN M P, LUNDGREN D G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans II: Manometric studies [J]. Journal of Bacteriology, 1959, 78(3): 326-331.
[28] ZHAO Hong-bo, WANG Jun, GAN Xiao-wen, HU Ming-hao, ZHANG Er-xing, QIN Wen-qing, QIU Guan- zhou. Cooperative bioleaching of chalcopyrite and silver- bearing tailing by mixed moderately thermophilic culture: An emphasis on the chalcopyrite dissolution with XPS and electrochemical analysis [J]. Minerals Engineering, 2015, 81: 29-39. DOI: 10.1016/j.mineng.2015.07.015.
(Edited by YANG Hua)
中文导读
精确调控搅拌浸出提高高硫金精矿的生物预氧化效率
摘要:来自于中国贵州的某高硫难处理金精矿含硫41.82%,含金15.12 g/t,其中82.11%的金被硫化物包裹,直接浸出金回收率低。针对该样品开展了生物预氧化搅拌浸出研究,同时对生物预氧化过程体系pH进行了在线调控。结果表明,生物氧化预处理可以大幅度提高高硫难处理金精矿的金回收率,在最适pH为1.3的条件下,pH调控工艺与无pH调控的工艺相比,硫的氧化率从79.31%提高到83.29%,黄金的回收率从76.54%增加到83.23%,在此条件下,混合菌可以保持较高的活性,同时可以避免黄钾铁矾的形成。生物预氧化渣浸金结果表明,对于高硫难处理金精矿,金的回收率与硫的氧化速率呈正相关,在一定范围内金的回收率随着硫氧化速率的增加而增加。
关键词:生物氧化;高硫难处理金精矿;搅拌浸出;pH调控
Foundation item: Projects(51704028, 51574036) supported by the National Natural Science Foundation of China; Project supported by Program for Key Laboratory of Biohydrometallurgy of Ministry of Education Foundation, China
Received date: 2019-06-25; Accepted date: 2020-04-07
Corresponding author: SHANG He, Senior Engineer; Tel: +86-10-60662775; E-mail: shanghe123321@163.com; ORCID: 0000-0002- 9281-6757

