
Leaching behavior of metals from limonitic laterite ore by high pressure acid leaching
GUO Xue-yi, SHI Wen-tang, LI Dong, TIAN Qing-hua
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 5 February 2010; accepted 8 April 2010
Abstract: The leaching behavior of metals from a nickeliferous limonitic laterite ore was investigated by high pressure acid leaching process for the extraction of nickel and cobalt. The effects of sulfuric acid added, leaching temperature, leaching time and liquid/solid (L/S) ratio on metals extraction were examined. More than 97% Ni, 96% Co, 93% Mn, 95% Mg and less than 1% Fe are extracted under optimum conditions. Analysis of the high pressure acid leaching residue by chemical and XRD analysis indicates that the residual iron and sulfur are mainly present in phases of hematite and alunite, respectively. The high pressure leaching process provides a simple and efficient way for the high recovery of nickel and cobalt from laterite ore, leaving residue as a suitable iron resource.
Key words: high pressure acid leaching; limonitic laterite; leaching behavior; nickel
1 Introduction
Nickel is a strategic metal with high impact strength and ductility properties and mainly used in the fields of stainless steel and nonferrous alloy production as well as electroplating and chemical industry, etc[1-2]. About two-thirds of the nickel is consumed by the stainless steel industry which has been growing at the rate of 5%-6% per annum over the last 20 years. The nickel reserved in oxide ores accounts for 65%, but now 60% of nickel is produced commercially from sulfide ores[3-4]. However, as the rapid development of industry and the decreasing reserves of sulfide ores, laterite is becoming a significant source for nickel. The reserve of nickel laterite in Philippines reaches 17.4% of the total reserves, ranking second in the world[5-6].
Hydrometallurgical processes are the main methods applied for the extraction of nickel and cobalt from nickeliferous laterite ores, which involve ammonia- ammonium carbonate leaching[7], sulfation-roasting- leaching[8], atmospheric acid leaching[9] and high pressure acid leaching[10-11]. McDONALD and WHITTINGTON[12] reviewed the processes reported in the past 30 years that can be used to extract nickel and cobalt with the focus on atmospheric acid leaching and high pressure acid leaching. At present, the high pressure acid leaching is the only industrial process for nickel extraction from laterite ore, which provides high recovery of nickel and cobalt, allows acceptable acid consumption and produces low residual iron in solution. The addition of alkali sulfates, especially Na2SO4 or Na2SO3, plays a significant role in catalyzing the acid leaching process[13-15].
In this work, the high pressure acid leaching is adopted to extract nickel and cobalt from laterite ore. The effects of various parameters including sulfuric acid added, leaching time and temperature and liquid/solid (L/S) ratio on leaching behaviors of nickel, cobalt and iron are explored. The leaching behaviors of manganese and magnesium are also investigated because of their important associations with nickel and cobalt extractions and downstream separation.
2 Experimental
2.1 Materials
The laterite ore come from the Tubay region, Mindanao, Philippines. The raw material used in this study was a typical limonitic laterite ore with high iron content. It was ground to size less than 180 μm for acid leaching. Table 1 shows the chemical composition of the sample. All chemical reagents used in this test are of analytical grade.
Table 1 Chemical composition of laterite ore (mass fraction, %)

In mineralogical characterization, X-ray diffraction pattern (XRD) was detected on a D/Max-2550 X-ray diffractometer (JEOL, Japan) with Cu Kα radiation from 10° to 85°. Scanning electron microscopy (SEM) images were obtained by using a JSM-6360LV spectrometer (Rigaku, Japan).
XRD (Fig.1) analysis of the laterite ore showed that the iron presents predominantly as FeOOH. SEM (Fig.2) image revealed the elliptic morphology of the laterite ore.

Fig.1 XRD pattern of laterite ore

Fig.2 SEM image of laterite ore
2.2 Experimental procedure
The high pressure acid leaching process was conducted in a 5 L Kunchang model vertical reactor (Kunchang of China) manufactured with titanium alloy and controlled to within ±1 °C using a temperature controller. Agitation at 300 r/min was provided by a magnetically driven twin impeller.
For all experiments, the required amount of sulfuric acid was added into the slurry which was prepared by mixing 800 g of ore and 5 g of Na2SO3 with a certain amount of water, and then the mixture was injected into the reactor before the temperature was raised to desired value. For sample collection, the sample was cooled to 85 °C by circle water bath after reaction and then it was pumped to a container. The sludge was filtered and the residue was dried overnight at 105 °C.
Elemental analysis of the residue samples and filtered leaching liquor was conducted using a Varian 720-ES ICP.
2.3 Principles
Nickel in laterite mainly exists as one part of goethite. To obtain a high recovery of nickel, the goethite must be completely destroyed. It has been reported that the goethite can be totally decomposed to iron oxide in acidic condition when the temperature is higher than 240 °C[10]. The main reactions are as follows:
FeOOH+3H+→Fe3++2H2O (1)
NiO+2H+→Ni2++H2O (2)
At the same time, relative reactions are as follows:
2Fe3++3H2O→Fe2O3+6H+ (3)
Fe3++SO42-+H2O→Fe(OH)SO4+H+ (4)
Based on the reactions (1) to (4), it is quite clear that the dissolution of iron minerals consumes huge amount of acid in the leaching process, and equal amount of acid is produced after the hydrolyzation of ferric ions at high temperature. Thus, the high pressure reactions transform the goethite into iron oxide without consumption of acid.
Cobalt mainly exists as manganese oxide in the form of Co2O3. In the leaching process, the manganese and cobalt were dissolved as the following reactions when Fe2+ and Cr3+ exist:
3MnO2+2H2O+2Cr3+→3Mn2++2H2CrO4 (5)
Co2O3+6H++2Fe2+→2Co2++3H2O+2Fe3+ (6)
Basically, the extraction of cobalt in laterite is easier than nickel because of the high content of iron and easy manganese dissolution in acid.
3 Results and discussion
3.1 Effect of sulfuric acid added on metal extraction
A series of high pressure leaching experiments were carried out by varying the addition of sulfuric acid from 125 kg/t ore to 400 kg/t ore at 250 °C with leaching time of 1 h and L/S ratio of 3:1(i.e. 3 L water and 1 kg ore). From the results shown in Fig.3, it can be found that the nickel extraction efficiency obviously increases with the increase of sulfuric acid added and reaches to 98% when the amount of acid added is up to 250 kg/t ore, and with further increase the effect is marginal. The extraction of manganese shows a similar extraction behavior as nickel. The extractions of cobalt and magnesium increase slowly with the increasing of sulfuric acid added, and the extraction efficiencies are more than 80% in the testing range. However, the addition of sulfuric acid has a significant effect on the iron dissolution, which increases from 0.15% to 2.70% as the amount of acid added increases. Consequently, further tests were carried out with the addition of sulfuric acid fixed at 250 kg/t ore.

Fig.3 Effect of addition of sulfuric acid on metal extractions
3.2 Effect of leaching temperature on metal extraction
The leaching temperature also plays a significant role in metals extraction. Fig.4 shows the effect of leaching temperature on metals extraction with acid addition of 250 kg/t, leaching time of 1 h and L/S ratio of 3:1.
It can be seen from Fig.4 that the extractions of nickel, cobalt, manganese and magnesium increase steadily with leaching temperature increasing to about 250 °C. Further increasing temperature has slight influences. It shows that leaching temperature has a considerable negative effect on the iron dissolution, and less than 1% Fe is leached out from the sample at 250 °C.
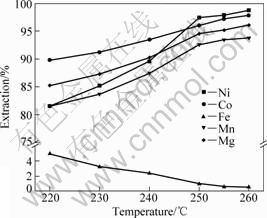
Fig.4 Effect of leaching temperature on metal extractions
Based on the above results, the optimum leaching temperature is fixed at 250 °C.
3.3 Effect of leaching time on metal extraction
Leaching processes with different time were carried out with acid addition of 250 kg/t, leaching temperature of 250 °C and L/S ratio of 3:1, and the results are shown in Fig.5. It is observed that the extractions of nickel and cobalt increase apparently with leaching time increasing up to 1 h. Further increasing has a marginal effect on nickel and cobalt extraction. The manganese and magnesium show similar leaching behaviors to nickel and cobalt. It can also be seen from Fig.5 that the iron dissolution decreases slowly after leaching for 40 min. Consideration must be given to the better extractions of nickel and cobalt as well as to the higher associated energy cost. Therefore, the optimum leaching time is fixed at 1 h.
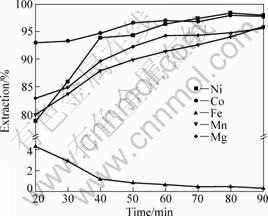
Fig.5 Effect of leaching time on metal extractions
3.4 Effect of L/S ratio on metal extractions
The effect of L/S ratio on metal extractions in leaching process is shown in Fig.6. It can be seen that the leaching efficiencies of nickel, cobalt, manganese and magnesium increase slightly as the L/S ratio increases. Meanwhile, the iron just shows an opposite leaching behavior to other metals. As L/S ratio ranges from 1.5:1 to 4:1, iron dissolution decreases from 2.92 g/L to 0.42 g/L, which is due to the decrease of H+ concentration in leaching solution. However, the decrease of iron dissolution in leaching solution by increasing L/S ratio is accompanied with the decrease of production capacity. So, the optimum L/S ratio is chose as 3:1.
3.5 Optimum conditions and residue characterization
Based on the results determined in the previous tests, the optimum conditions are determined as the follows: sulfuric acid added of 250 kg/t ore, leaching temperature of 250 °C, leaching time of 1 h and L/S ratio of 3:1. The leaching efficiencies of nickel, cobalt, iron, manganese
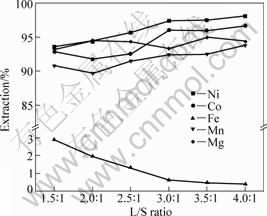
Fig.6 Effect of L/S ratio on metal extractions
and magnesium under optimum conditions are 97%, 96%, 0.8%, 93% and 95%, respectively.
The chemical composition of the residue obtained in high pressure acid leaching process under optimum conditions is shown in Table 2. The residue was also characterized by XRD technique and the result is shown in Fig.7. From Table 2, it is clear that the iron content in leaching residue is more than 50% which can be recycled as the form of hematite(Fe2O3). Both nickel and cobalt in residue are lower than 0.1%. From Fig.7, it can be seen that the main phases present in the residue include hematite and alunite [NaAl3(OH)6(SO4)3]. The precipitation of alunite in the leaching process leads to a high content of sulfur in the leaching residue. After the
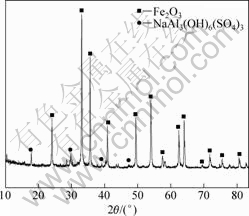
Fig.7 XRD pattern of leaching residue
Table 2 Elemental composition of residue obtained under optimum conditions (mass fraction, %)
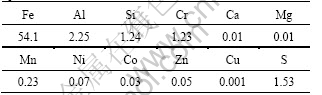
sulfur is decreased or even removed, the leaching residue would be an eligible raw material for a steel production company.
4 Conclusions
1) The high pressure acid leaching process extracts more than 97% Ni, 96% Co, 93% Mn and 95% Mg and less than 1% iron from a nickeliferous laterite ore, and its extraction efficiency is mainly influenced by the sulfuric acid added, the leaching temperature and time and the L/S ratio.
2) The optimum conditions are determined as the sulfuric addition of 250 kg/t ore, leaching temperature of 250 °C, leaching time of 1 h and L/S ratio of 3:1.
3) Chemical analysis and XRD technique were adopted for the characterization of the leaching residue which shows a promising application as the raw material in steel production company.
References
[1] SUDOL S. The thunder from down under: everything you wanted to know about laterites but were afraid to ask [J]. Canadian Mining Journal, 2005, 126(5): 8-12.
[2] CHANG Yong-feng, ZHAI Xiu-jing, FU Yan. Phase transformation in reductive roasting of laterite ore with microwave heating [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 969-973.
[3] ANTHONY M T, FLETT D S. Nickel processing technology: A review [J]. Minerals Industry International, 1997, 1: 26-42.
[4] LIU Da-xing. Recent development in nickel and cobalt recovery technologies from laterite [J]. Nonferrous Metal, 2002, 3: 6-10. (in Chinese)
[5] ZHU Jing-he. Exploration laterite-nickel ore and analysis on utilization technology [J]. World Nonferrous Metals, 2007, 10: 7-9. (in Chinese)
[6] XU Qing-xin. The past and the future of nickel laterites [J]. China Nonferrous Metallurgy, 2005, 12(6): 1-8. (in Chinese)
[7] VALIX M, CHEUNG W H. Effect of sulfur on the mineral phases of laterite ores at high temperature reduction [J]. Minerals Engineering, 2002, 15: 523-530.
[8] GUO Xue-yi, LI Dong, PARK K H. Leaching behavior of metals from a limonitic nickel laterite using a sulfation-roasting-leaching process [J]. Hydrometallurgy, 2009, 99: 144-150.
[9] CHANDER S. Atmospheric pressure leaching of nickeliferous laterites in acidic media [J]. Transactions of the India Institute of Metals, 1982, 35: 366-371.
[10] GEORGIOU D, PAPANGELAKIS V G. Sulfuric acid pressure leaching of a limonitic laterite: chemistry and kinetics [J]. Hydrometallurgy, 1998, 49: 23-46.
[11] RUBISOV D H, PAPANGELAKIS V G. Sulfuric acid pressure leaching of laterites—A comprehensive model of a continuous autoclave [J]. Hydrometallurgy, 2000, 58: 89-101.
[12] McDONALD R G, WHITTINGTON B I. Atmospheric acid leaching of nickel laterites review Part I. Sulfuric acid technologies [J]. Hydrometallurgy, 2008, 91: 35-55.
[13] KOLTA G A, ASKAR M H. Thermal decomposition of some metal sulphates [J]. Thermochimica Acta, 1975, 11: 65-72.
[14] KAR B B, SWAMY Y V. Some aspects of nickel extraction from chromitiferous overburden by sulphatization roasting [J]. Minerals Engineering, 2000, 13: 1635-1640.
[15] FU Chong-shui. Theory of non-ferrous metallurgy [M]. Beijing: Metallurgical Industry Press, 1993: 103-105. (in Chinese)
镍红土矿高压酸浸过程的金属元素浸出行为
郭学益,石文堂,李栋,田庆华
中南大学 冶金科学与工程学院,长沙 410083
摘 要:以镍、钴的提取为目的,研究褐铁矿型镍红土矿高压酸浸过程中各金属元素的浸出行为,探讨硫酸加入量、浸出温度、浸出时间及液固比对各金属元素浸出率的影响。实验结果表明,在优化条件下Ni、Co、Mn和Mg的浸出率分别达到97%、96%、93%和95%以上,则Fe的浸出率小于1%。对高压浸出渣的分析表明,渣中的铁和硫主要分别以赤铁矿和明矾石的形式存在。高压浸出过程提供了一种从镍红土矿中提取镍和钴的简单有效的方法,同时产生具有利用价值的铁渣。
关键词:高压酸浸;褐铁矿型红土矿;浸出行为;镍
(Edited by LAI Hai-hui)
Corresponding author: GUO Xue-yi; Tel: +86-731-88877863; Fax: +86-731-88836207; E-mail: xyguo@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(11)60698-5