Ag/介孔CeO2纳米复合材料的合成、表征及抗菌性能
来源期刊:中国有色金属学报(英文版)2012年第6期
论文作者:陆晓旺 钱君超 陈丰 李霞章 陈志刚
文章页码:1418 - 1422
关键词:CeO2;介孔;银纳米颗粒;抗菌活性
Key words:CeO2; mesoporous; silver nanoparticle; antibacterial activity
摘 要:利用改进的溶剂挥发诱导自组装法(EISA)制备高比表面积介孔CeO2,通过改进的乙二醇还原法,在得到的介孔CeO2中负载上不同量的银。采用粉末X射线衍射(XRD)、透射电子显微镜(TEM)、能谱仪(EDS),N2吸附-脱附法方法对产物进行表征。由BJH方程计算得到材料的孔径分布,以BET法计算材料的比表面积。研究负载不同比例银纳米粒子的介孔CeO2的结构及其抗菌活性。实验表明所制备的新材料具有很好的抗菌性能。
Abstract: Mesoporous CeO2 with high specific surface area was synthesized using a modified evaporation-induced self-assembly (EISA) method, and a series of different amounts of Ag were loaded to this mesoporous CeO2 by a modified ethylene glycol reduction route. The samples were characterized by powder X-ray diffraction (XRD), transmission electron microscopy (TEM), energy-dispersive spectrometry (EDS), nitrogen adsorption-desorption, Brunauer–Emmett–Teller (BET) and Barrett-Joyner-Halenda (BJH) methods. The mesoporous CeO2 structure with different proportions of silver nanoparticles and its antibacterial activity were adequately studied, confirming that obtained novel materials show a good antibacterial effect.

![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1418-1422
LU Xiao-wang1,2, QIAN Jun-chao3, CHEN Feng3, LI Xia-zhang1, CHEN Zhi-gang4
1. School of Materials Science and Engineering, Changzhou University, Changzhou 213164, China;
2. Key laboratory of Advanced Metallic Materials of Changzhou City, Changzhou 213164, China;
3. School of Materials Science and Engineering, Jiangsu University, Zhenjiang 212013, China;
4. School of Chemistry and Bioengineering, Suzhou University of Science and Technology, Suzhou 215011, China
Received 13 June 2011; accepted 30 September 2011
Abstract: Mesoporous CeO2 with high specific surface area was synthesized using a modified evaporation-induced self-assembly (EISA) method, and a series of different amounts of Ag were loaded to this mesoporous CeO2 by a modified ethylene glycol reduction route. The samples were characterized by powder X-ray diffraction (XRD), transmission electron microscopy (TEM), energy-dispersive spectrometry (EDS), nitrogen adsorption-desorption, Brunauer–Emmett–Teller (BET) and Barrett–Joyner– Halenda (BJH) methods. The mesoporous CeO2 structure with different proportions of silver nanoparticles and its antibacterial activity were adequately studied, confirming that obtained novel materials show a good antibacterial effect.
Key words: CeO2; mesoporous; silver nanoparticle; antibacterial activity
1 Introduction
CeO2 is an important rare earth oxide and has potential applications in areas including catalysis sorption [1,2], polishing agents [3,4], photonic and electronic devices [5-7], gas sensing [8] and solid oxide fuel cells (SOFC) [9,10]. Many metallic and non-metallic elements have been tried, in the past, to be implanted into ceria to improve its catalytic activity [11,12], but few papers concerned with the antibacterial property of nanoparticles implanted in the ceria. Generally, the properties of ceria could be improved from two aspects, structural modification and element decoration.
To achieve higher specific surface area desirable for better applications, several efforts have been made to fabricate mesoporous CeO2. Mesoporous CeO2 is of extremely high surface activity and well oxygen storage capacity due to its porous structure, which can adsorb various ions easily in its pores and on its surfaces. Recently, CORMA et al [13] have synthesized mesostructured CeO2 materials using CeO2 nanoparticles as starting materials and a EO20PO70EO20 triblock copolymer as the soft template. ROSSINYOL et al [14] have recently applied SBA-15 and KIT-6 mesoporous silica as the hard template to synthesizing mesoporous ceria.
In order to further improve the properties of CeO2, various metal nanoparticles on CeO2 support were used, such as Pt, Rh, Ru, Au and Ag [15-20]. Silver is known to have a wide antibacterial spectrum and relatively high safety. When silver was supported onto inorganic carrier and released from the surface slowly by design, it acted as inorganic disinfectant, which is superior in terms of safety, durability and heat resistance [21,22]. Herein, the synthesis of mesoporous CeO2 using CH3(CH2)15N(CH3)3Br as a soft temple to support the different amounts of Ag on them was reported, the antibacterial activity of the samples was investigated.
2 Experimental
2.1 Preparation of mesoporous CeO2
Typically, 0.005 mol of cetyltrimethylammonium bromide (CH3(CH2)15N(CH3)3Br, referred as CTAB, SCRC) was first dissolved in a solution of 15 mL ethanol, then 0.01 mol of cerium acetate hydrate(Sigma-Aldrich) and 0.001 mol citric acid monohydrate(C6H8O7·H2O, SCRC) dissolved in 5 mL deionized water were added. The mixture was stirred for 2 h to ensure thorough mixing, and subsequently dispersed with a micropipette onto Petri dishes (100 mm in diameter), and was then placed in an oven to form a gel at 40 ℃. After 48 h aging, the gel was dried at 80 ℃ for 24 h. Calcination was carried out by slowly increasing temperature from room temperature to 400 ℃ at 2 ℃/min ramping rate and kept at 400 ℃ for 5 h.
2.2 Preparation of silver-supported mesoporous CeO2
Silver-supported mesoporous CeO2 material was prepared by a modified ethylene glycol(EG) reduction route. 0.5 g mesoporous CeO2 was dispersed in 100 mL of EG to form a mixture in a three-necked flask. Then a given amount of PVP (Sigma-Aldrich, Mr=29000) and Ag(NO)3 (the molar ratio of PVP to Ag+ was 5:1) were added. The flask was sealed and then pure N2 was insufflated to replace the air. Subsequently, such a mixture was stirred and maintained at a refluxing temperature of 80 ℃ for 10 h in the dark. After cooling to room temperature, the mixture was filtrated, washed copiously with water and ethanol and dried under vacuum at 60 ℃ for 12 h then calcined at 300 ℃ for 5 h.
2.3 Antibacterial property measurement
To make a suspension, sterilized CeO2 (500 mg) powder was added into 100 mL sterile water with mild sonication, and sterilized pure mesoporous CeO2, 1% Ag/CeO2, 3% Ag/CeO2, 5% Ag/CeO2 powders (500 mg) were separately added into 100 mL steriled water with mild sonication. The standard gram-negative bacteria E. coli (ATCC 25922) was inoculated into lactose broth (LB) and cultured aerobically at 37 ℃ for 24 h. Then 1mL aliquots of bacterial inocula were added into four 12 cm-diameter LB agar Petri-dishes, followed by the addition of four kinds of 3 mL CeO2 powder suspension prepared hereinbefore to four Petri-dishes. The mixed suspension was put horizontally in the dish and cultured at 37 ℃ for 48 h. The growth of the bacterium on the dishes was observed by counting the number of colony.
2.4 Material characterization
The crystal structures of the samples were determined by a powder X-ray diffraction on a Rigaku D/max-RB diffractometer using Cu Kα radiation (λ=0.15406 nm). The XRD data were recorded for 2θ values between 10° and 80° with a 0.02° step size. Transmission electron microscopy (TEM) and energy- dispersive spectrometry (EDS) were taken for morphology and chemical composition by JEOL JEM-2010 transmission electron microscope, which was operated at 200 kV. The textural properties and porosity of samples were studied by adsorption of nitrogen at 77 K with a Micromeritics ASAP-2010C instrument. Surface areas were calculated by the BET method and pore size distribution was analyzed using the BJH method.
3 Results and discussion
The XRD patterns of mesoporous CeO2 and silver- supported mesoporous CeO2 are illustrated in Fig. 1. The diffraction peaks at 2θ=28.5°, 33.0°, 47.4° and 56.4° can be indexed to a cubic structure (space group Fm3m, JCPD NO43—1002)with lattice space α=0.541 nm. The XRD patterns of 1%, 3%, 5% Ag-supported mesoporous CeO2, show only discernable diffraction peaks at 2θ=38.1°, 44.3°, which could be assigned to the metal silver(JCPD NO65—2871). The EDS spectrum in Fig. 2 shows 3% Ag-supported mesoporous CeO2, suggesting that only Ce, O and Ag are detected.
Figures 3(a), (b) show the TEM images of 3% Ag- supported mesoporous CeO2. The figures reveal that the sample is not organized [14] as well as that in the case of CeO2 and consists of porous with diameter of 5-10 nm. The Ag nanoparticles are encapsulated by CeO2 meso-structure and randomly distributed throughout the entire mesoporous CeO2 framework. The CeO2 meso-structure avoids a severe breakdown throughout the Ag nanoparticles supporting step. In Fig. 3(b), many different lattice fringes can be found which allows for identification of the crystallographic spacings of CeO2 and Ag. The fringes of d0.317 nm match the (111) crystallographic planes of CeO2, while the fringes of d0.245 nm match the (111) planes of the metal Ag.

Fig. 1 XRD patterns of mesoporous CeO2 and silver-supported mesoporous CeO2
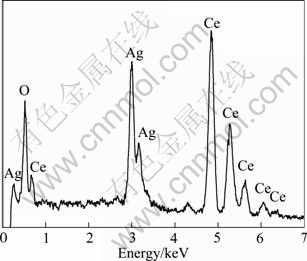
Fig. 2 EDS patterns of 3% Ag-supported mesoporous CeO2
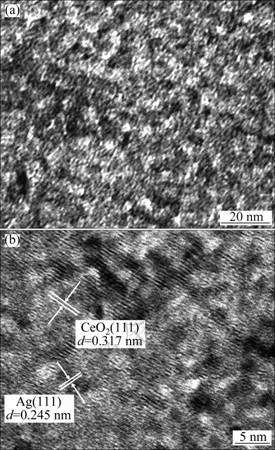
Fig. 3 TEM images of 3% Ag-supported mesoporous CeO2
With CTAB as a surfactant and citric acid as a complexing agent, the mesostructure of CeO2 was fabricated by the evaporation-induced self-assembly (EISA) process. After Ag(NO)3 and PVP were added to the mesoporous CeO2 ethylene glycol solution, on the pore of mesoporous CeO2, Ag+ precursors were reduced with ethylene glycol to form silver atoms, then these silver atoms nucleated and grew into silver nanostructures. The whole reaction can be described by the following equations:
CH2OH—CH2OH→CH3CHO + H2O (1)
CH3CHO+2Ag++2OH-→CH3COOH+2Ag+H2O (2)
Figure 4 shows the nitrogen adsorption-desorption isotherms of the mesoporous CeO2 and Ag-supported mesoporous CeO2. According to IUPAC classification, the similar nitrogen adsorption-desorption isotherms of all samples can be classified as a type IV isotherm, and each hysteresis loop is H2 type, typical of a mesoporous material. The nitrogen adsorption-desorption measurements of the mesoporous CeO2 and Ag- supported mesoporous CeO2 show a similar isotherm with a jump at a relative pressure of 0.3-0.5 that corresponds to the capillary condensation of the mesoporous and another jump at a relative pressure of 0.8-0.9, reflecting the textural pores between the particles. The pore size distributions are shown in Fig. 5, and the pore size of the samples is 5-7 nm. The BET specific surface area of the sample is summarized in Table 1. With the silver loaded amount increasing, the pore size and the BET specific surface area all decrease. This means that the Ag nanoparticles have incorporated into the pores of the mesoporous CeO2.

Fig. 4 Nitrogen adsorption-desorption isotherms of mesoporous CeO2 and Ag-supported mesoporous CeO2
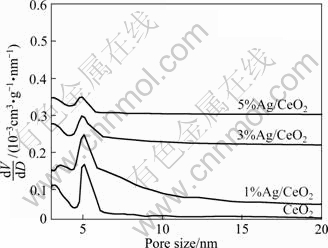
Fig. 5 Pore size distribution of mesoporous CeO2 and Ag- supported mesoporous CeO2
Table 1 BET specific surface area of mesoporous CeO2 and Ag-supported mesoporous CeO2

4 Antibacterial activity
The mixed suspension was put horizontally in the dish and cultured at 37 ℃ for 48 h. The growth of the bacterium on the dishes was observed by counting the number of colony. The antimicrobial efficiency of the samples was tested against gram negative bacterium E. coli. Figure 6 shows four culture dishes with and without antibacterial agents after antibacterial test. In Fig. 6, no antibacterial activity was detected in the culture plate with pure mesoporous CeO2 powder. After 24 h of the antibacterial test, the E. coli colonies number reached over 500. 48 h later, thick white lawn appeared on or around the dish. 1% Ag/CeO2 shows little antibacterial activity because Ag+ ions leading to inhibition of bacterium do not operate at such a low level of concentration.
Figure 6 shows that the antibacterial propriety of the suspension scattered on the dish is strong. The CeO2 powders loaded with the silver nanoparticles significantly retard the bacteria growth. Ag nanoparticles have an appreciable effect on bacteria killing, as shown in Fig. 6 (3% Ag/CeO2), and the E. coli colony number does not exceed 50 after 24 h. This exhibits that Ag nanoparticles can endow CeO2 powders with excellent antibacterial properties. Yet, the proportion of Ag doped in the materials is still inadequate. Therefore, a few colonies appear on the dishes. Figure 6 (5% Ag/CeO2) shows that the antibacterial propriety of the suspension scattered on the dish is strong. The CeO2 powders loaded with the silver nanoparticles (5%Ag/CeO2) entirely retard bacteria growth.

Fig. 6 Antibacterial properties of pure mesoporous CeO2 and Ag-supported mesoporous CeO2 against E. coli
This good antibacterial activity is possibly due to Ag ions eluted from Ag-loaded mesoporous CeO2 surface, which could be absorbed onto the surface of bacteria cells with damaging the cell membrane and solidifying structure of proteins structure. It will lead to a distortion of E. coli cell shape and give rise to leakage of the intracellular constituents, resulting in the death of the bacteria. The oxygen species released from the decomposition of Ag2O participated in the oxidation of hydroxide ion, and the reoxidation of Ag to Ag2O would be achieved via the oxygen species from mesoporous CeO2. Mesoporous CeO2, which possesses high surface area and well oxygen storage capacity, will interact with the oxygen molecules in the reaction stream through the cycle of Ce4+/Ce3+ [23], maintaining the recycling of the oxidative state of the silver, as shown in Fig. 7. This excellent interface is thought to promote the formation of the reactive Ag ions. Based on this study it is possible to use this sort of powder as a promising bioactive antibacterial material.
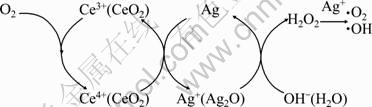
Fig. 7 Schematic illustration of oxygen transfer mechanism
5 Conclusions
1) Mesoporous CeO2 was synthesized using a modified evaporation-induced self-assembly method, different amounts of Ag were loaded on this mesoporous CeO2 by a modified ethylene glycolreduction route. The obtained materials have a high surface area and uniform pore size.
2) When 5% Ag was loaded on the mesoporous CeO2, the antibacterial property shows that the sample has a considerable bactericidal activity, due to the synergistic effect between the silver nanoparticles and mesoporous CeO2.
References
[1] MURUGAN B, RAMASWAMY A V. Defect-site promoted surface reorganization in nanocrystalline ceria for the low-temperature activation of ethylbenzene [J]. J Am Chem Soc, 2007, 129: 2062-2063.
[2] ZHANG D S, PAN C S, SHI L Y, HUANG L, FANG J H, FU H X. A highly reactive catalyst for CO oxidation: CeO2 nanotubes synthesized using carbon nanotubes as removable templates [J]. Microporous Mesoporous Mater, 2009, 117: 193-200.
[3] KOSYNKIN V D, ARZGATKINA A A, IVANOV E N, CHTOUTSA M G, GRABKO A I, KARDAPOLOV A V, SYSINA N A. The study of process production of polishing powder based on cerium dioxide [J]. J Alloys Compd, 2000, 303-304: 421-425.
[4] HOSHINO T, KURATA Y, TERASKI Y, SUSA K. Mechanism of polishing of SiO2 films by CeO2 particles [J]. Non-Cryst Solids, 2001, 283: 129-136.
[5] AVELLANEDA C O, BERTON M A C, BULHOESL O S. Optical and electrochemical properties of CeO2 thin film prepared by an alkoxide route [J]. Sol Energy Mater Sol Cells, 2008, 92: 240-244.
[6] PATSALAS P, LOGOTHETIDIS S, METAXA C. Optical performance of nanocrystalline transparent ceria films [J]. Appl Phys Lett, 2002, 81: 466-468.
[7] ?ZER N. Optical properties and electrochromic characterization of sol–gel deposited ceria films [J]. Sol Energy Mater Sol Cells, 2001, 68: 391-400.
[8] IZU N, SHIN W, MURAYAMA N, KANZAKI S. Resistive oxygen gas sensors based on CeO2 fine powder prepared using mist pyrolysis [J].Sens Actuators B, 2002, 87(1): 95-98.
[9] LAOSIRIPOJANA N, SANGTONGKITCHAROEN W, ASSABUMRUNGRAT S. Catalytic steam reforming of ethane and propane over CeO2-doped Ni/Al2O3 at SOFC temperature: Improvement of resistance toward carbon formation by the redox property of doping CeO2 [J]. Fuel, 2006, 85(3): 323-332.
[10] SUN C W, XIE Z, XIA C R, LI H. CHEN L Q. Investigations of mesoporous CeO2–Ru as a reforming catalyst layer for solid oxide fuel cells [J]. Electrochem Commun, 2006, 8(5): 833-838.
[11] HU C Q, ZHU Q S, JIANG Z, ZHANG Y Y, WANG Y. Preparation and formation mechanism of mesoporous CuO–CeO2 mixed oxides with excellent catalytic performance for removal of VOCs [J]. Microporous Mesoporous Mater, 2008, 113(1-3): 427-434.
[12] MAO C J, ZHAO Y X, QIU X F, ZHU J J, BURDA C. Synthesis, characterization and computational study of nitrogen-doped CeO2 nanoparticles with visible-light activity [J]. Phys Chem Chem Phys, 2008, 10: 5633-5638.
[13] CORMA A, ATIENZAR P, GARCLA H, CHANE-CHING J Y. Hierarchically mesostructured doped CeO2 with potential for solar-cell use [J]. Nat Mater, 2004, 3: 394-397.
[14] ROSSINYOL E, ARBIOL J, PEIR? F, CORNET A, MORANTE J R, TIAN B. Nanostructured metal oxides synthesized by hard template method for gas sensing applications [J]. Sens Actuators B, 2005, 109(1): 57-63.
[15] BARRAB?S N, DAFINOV A, MEDINA F, SUEIRAS J E. Catalytic reduction of nitrates using Pt/CeO2 catalysts in a continuous reactor [J]. Catal Today, 2010, 149(3-4): 341-347.
[16] CAI W J, WANG F G, VAN V A C, PROVENDIER H, MIRODATOS C, SHEN W J. Autothermal reforming of ethanol for hydrogen production over an Rh/CeO2 catalyst [J]. Catal Today, 2008, 138: 152-156.
[17] HOSOKAWA S, KANAI H, UTANI K, TANIGUCHI Y, SAITO Y, IMAMURA S. State of Ru on CeO2 and its catalytic activity in the wet oxidation of acetic acid [J]. Appl Catal B, 2003, 45(3): 181-187.
[18] FU Q, SALTSBURG H, FLYTZANI S M. Active non-metallic Au and Pt species on ceria-based water-gas shift catalysts [J]. Science, 2003, 301: 935-939.
[19] CARRETTIN S, CONCEPCION P, COMA A, L?PEZ NIETO J M, PUNTES V F. Nanocrystalline CeO2 increases the activity of Au for CO oxidation by two orders of magnitude [J]. Angew Chem Int Ed, 2004, 43: 2538-2540.
[20] IMAMURA S, YAMADA H, UTANI K. Combustion activity of Ag/CeO2 composite catalyst [J]. Applied Catalysis A, 2000, 192(2), 221-226.
[21] TOP A, ?LK? S. Silver, zinc, and copper exchange in a Na-clinoptilolite and resulting effect on antibacterial activity [J]. Appl Clay Sci, 2004, 27(1-2): 13-19.
[22] RAVINDRA S, MURALI M Y, REDDY N N, RAJU K M. Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via “green approach” [J]. Colloids Surf A, 2010, 367(1-3): 31-40.
[23] TANG X F, CHEN J L, LI Y G, LI Y, XU Y D, SHEN W J. Complete oxidation of formaldehyde over Ag/MnOx–CeO2 catalysts [J]. Chem Eng J, 2006, 118(1-2): 119-125.
陆晓旺1, 2,钱君超3,陈 丰3,李霞章1,陈志刚4
1. 常州大学 材料科学与工程学院,常州 213164;
2. 常州市先进金属材料重点实验室,常州 213164;
3. 江苏大学 材料科学与工程学院,镇江 212013;
4. 苏州科技学院 化学与生物工程学院,苏州 215011
摘 要:利用改进的溶剂挥发诱导自组装法(EISA)制备高比表面积介孔CeO2,通过改进的乙二醇还原法,在得到的介孔CeO2中负载上不同量的银。采用粉末X射线衍射(XRD)、透射电子显微镜(TEM)、能谱仪(EDS),N2吸 附-脱附法方法对产物进行表征。由BJH方程计算得到材料的孔径分布,以BET法计算材料的比表面积。研究负载不同比例银纳米粒子的介孔CeO2的结构及其抗菌活性。实验表明所制备的新材料具有很好的抗菌性能。
关键词:CeO2;介孔;银纳米颗粒;抗菌活性
(Edited by LI Xiang-qun)
Foundation item: Projects (21071107, 51002016) supported by the National Natural Science Foundation of China; Project (JQ201003) supported by Changzhou University Fund for Young Talent, China
Corresponding author: LU Xiao-wang; Tel: +86-519-86330066; Fax: +86-519-86330066; E-mail: lxwjpu@126.com
DOI: 10.1016/S1003-6326(11)61335-6

