Article ID: 1003-6326(2005)06-1401-06
Preparation of colloidal Sb2O5 from arsenic-alkali residue
CHAI Li-yuan(柴立元), WANG Jian-qiang(王建强),
WANG Yun-yan(王云燕), ZHENG Jun-chao(郑俊超)
(School of Metallurgical Science and Engineering, Central South University,
Changsha 410083, China)
Abstract: The stable colloidal antimony pentoxide was prepared by oxidation of the mixture of Sb2O3 and Sb2O5 obtained from arsenic-alkali residue by hydrometallurgical process, with hydrogen peroxide as oxidant and phosphoric acid as stabilizer. Effects of main factors were investigated. The theories on thermodynamics, kinetics and electrical double layer(EDL) were used to analyze the experimental phenomena and results. The results show that no aging time is the most beneficial to forming colloid, when molar ratio of phosphoric acid to antimony is in the range from 0.8 to 1.0 and 1.0 to 1.3, the particle sizes of sol with the concentration of 10% and 15% antimony pentoxide by mass are both smaller. With increasing concentration of the mixture of antimony oxide from 10% to 20%, the reaction time decreases from 90 to about 30min, but the optimized range of molar ratio of H3PO4 to antimony increases. The reaction temperature is not the main factor on particle size with the existence of H3PO4 in the temperature range from 60 to 90℃.
Key words: colloidal antimony pentoxide; arsenic-alkali residue; solid waste; comprehensive utilization CLC
number: TQ135.3 Document code: A
1 INTRODUCTION
Antimony compounds are commonly used as flame retardants. Conventional antimony compounds such as antimony trioxide are of a big particle size, resulting in low chemical activity, poor combination between antimony compounds and polymer, worse flame-resistant performance and the decrease of luster of polymer[1-5]. The way to overcome these shortages is to decrease the particle size. And the colloidal antimony pentoxide is desirable for this purpose, because of small particle size and the excellent properties of colloidal antimony pentoxide, such as better chemical activity, large specific surface area, high dispersion and heat-stability, less smoking amount, easy addition and combination to the polymer. Therefore, it was considered the best one among the antimony compounds[6-9] and has been widely applied in the industrial fields of fibre, plastic, dope, latex, paper and so on.
The methods for preparing colloidal antimony pentoxide, presently, were mainly developed, including refluxing oxidation[10-16], ion exchange[4], electrodialysis and colloidal chemical method[17, 18], in which refluxing oxidation was applied frequently, with HNO3, H2O2 and (NH4)2S2O8 used as oxidant, and pure antimony as raw material. However, there existed some shortages including no stability of present technologies.
In addition, more than 107 kg arsenic-alkali residues were produced annually in antimony metallurgical process in China, in which over 30% antimony exists as the phases of Na3SbO3, Na3SbO4 and Sb, and 1%-2% arsenic as the phases of Na3AsO3 and Na3AsO4. The stack of arsenic-alkali residue has resulted in the serious waste of antimony resource and horrible environmental pollution, limiting the sustainable development of the producer, and the poisonous accidents due to arsenic-alkali residue, have been paid great attention to by the Chinese government and the locality.
Based on the purpose of protecting environment and recycling the resource of antimony, therefore, a novel technology to treat arsenic-alkali residue was put forward for preparing colloidal Sb2O5 with small particle size and narrow distribution, including water leaching, acid leaching, hydrolyzing and preparing colloid. And the effects of aging time of mixtures after hydrolyzing, amount of phosphoric acid, concentration of mixtures and reaction temperature on the process of colloid formation and colloidal particle size were also studied, which was aimed at providing a new feasible technology for preparing qualified colloidal Sb2O5 by reusing arsenic-alkali residue.
2 EXPERIMENTAL
2.1 Preparation of colloidal antimony pentoxide
Arsenic-alkali residue from Hunan Tin Mine Co. Ltd., with antimony 30%, arsenic 1%-2%, was leached by water to separate antimony from arsenic. The leached residue without arsenic was further leached by hydrochloride, by which the solution was hydrolyzed to form the mixture of 70% Sb2O3 and 30% Sb2O5 as the raw material for preparing colloidal Sb2O5. Using refluxing oxidation and colloidal chemical method, both Sb2O3 and Sb2O5 can be transformed to colloidal Sb2O5. The aging time of the mixture solution was investigated on the effect of colloidal preparation. With centrifugal filtrating, H2O and H3PO4 were added to the mixture of antimony oxide in turn, and then, hydrogen peroxide was added into the mixture after being stirred and heated for some time. During the process, H3PO4 with volume concentration of 85% is used as stabilizer and H2O2 with the concentration of 30% is used as oxidant. The ratio of H3PO4 to antimony and the reaction temperature were also studied. Under the conditions of stirring, heating and refluxing, at the concentration of antimony pentoxide of about 0.186% and temperature of 20℃, clear and transparent colloid was formed.
2.2 Characteristics of colloidal antimony pentoxide
The colloidal antimony pentoxide was measured by D/MAX-RB XRD apparatus for the phase analysis, under the conditions of CuKα radiation 40kV, 50mA, step 0.02°, scanning rate 2(°)/min, scanning scope from 10°-90°. The colloidal particle size was analyzed by DELSA 440SX made in USA Coulter Company.
3 RESULTS AND DISCUSSION
3.1 Effect of aging time of mixture on formation of colloidal antimony pentoxide
The mixture of Sb2O3 and Sb2O5 was prepared by hydrolyzing the solution containing antimony. It is found through the experiments that aging time of the mixture strongly affects the formation of colloid. Fig.1 shows the XRD pattern of the mixture of antimony oxide without aging, Fig.2 shows the XRD pattern of the mixture aged for 4h, and Fig.3 shows the XRD pattern of the colloidal Sb2O5 prepared with amorphous mixture of antimony oxide without aging.
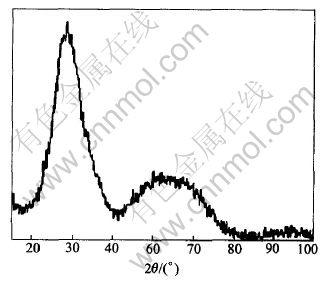
Fig.1 XRD pattern of mixture of antimony oxide without aging
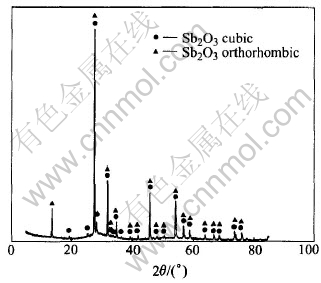
Fig.2 XRD pattern of mixture of antimony oxide with aging
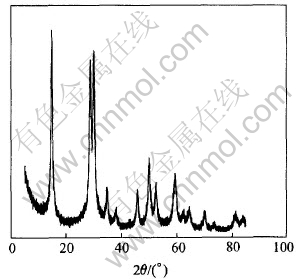
Fig.3 XRD pattern of colloidal Sb2O5
It can be seen from Figs.1 and 3 that the structure of mixture without aging is amorphous, and colloidal antimony pentoxide can be prepared from this amorphous mixture of antimony oxide. However, after aged for 4h, the main component of the mixture becomes cubic Sb2O3 and the minority is orthorhombic Sb2O3 from Fig.2, it fails to form colloid Sb2O5 using this mixture. It is concluded that aging has strong effect on the structure of the mixture of antimony oxide, and the structure of the mixture of antimony oxide has strong effect on forming colloid Sb2O5 too. So aging of the mixture strongly affected the formation of colloid, no aging is profitable on forming colloid Sb2O5.
According to crystal theory[19, 20], the structure and the energy of interface between core and substrate play the predominant roles in the process of heterogeneous nucleation. With the presence of substrate, the nucleation energy barrier and critical radius are reduced and it is easier to form steady crystal nucleus. Amorphous mixture of antimony oxide appears the properties of small particle, large specific surface area, plenty of surface activate plots and high free energy and capacity of surface absorption, so Sb2O5 embryos precipitating from solution are adsorbed on the surface of substrate instantly and grow into the stable colloidal particles rapidly, and this process of phase transformation is heterogeneous nucleation. At the same time, the concentration of Sb2O5 near the surface activate plots is higher than the other places in the solution, which is profitable for the diffusion of Sb2O5 molecule and growing of embryos. Besides, the particle size of amorphous mixture is small, which causes the increase of solubility of small particle and makes supersaturation of Sb2O5 higher, which makes the precipitating faster. Conversely, the aged mixture has few surface activate plots and crystal lacuna, which makes the nucleation place decrease and the new embryos dissolve without nucleation, so it is impossible to form plenty of crystal nucleus and further to form colloid.
3.2 Effect of amount of H3PO4 on particle size of colloidal Sb2O5
Hydrated Sb2O5 shows positive electricity in the water because of ionization, it is necessary to add stabilizer with negative electricity in the water to turn colloid of unstable thermodynamics state to the dynamics stable state, such as H3PO4[21], water-soluble alkanol amine[22], can be used as the stabilizer. Since there is few phosphoric acid consumption as stabilizer and it is a kind of flame retardant too, it is sure to use H3PO4 as stabilizer in the research. H3PO4 can decrease the activation energy of reaction, improve the reaction speed, suppress the growing up of the colloidal particle and contribute to getting colloidal antimony pentoxide with small particles and narrow distribution. Tan et al[11] and Zhang et al[9] have studied the effect of adding order of oxidant and stabilizer on particle size of colloidal Sb2O5, an unanimous conclusion has been drawn, that it is adding stabilizer firstly was better than adding oxidant firstly.
The H3PO4 as a stabilizer was added into the mixture solution, and subsequently the oxidants. When the concentration of antimony pentoxide exceeds its solubility, the nuclear embryos appear in the solution, the surface will be wrapped up and covered by the stabilizer at once and double electricity layer forms, hindering the particle growth and forming colloidal antimony pentoxide with small particles and narrow distribution. The effects of H3PO4 amount on the colloidal particles size were studied at the colloidal concentrations of 15% and 10% by mass, and the results are shown in Fig.4.
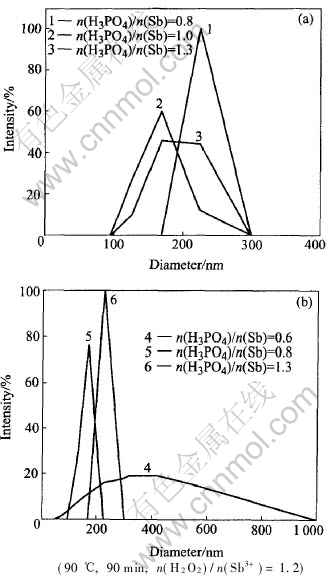
Fig.4 Effect of amount of stabilizer on particle size of colloidal Sb2O5 with concentration of 15%(a) and 10%(b)
It can be seen from Fig.4 that the colloidal particle size is affected by the amount of H3PO4. The average particle size of the colloidal Sb2O5 with the concentration of 15% are (225±19)nm, (164±32)nm and (189±37)nm, respectively at the molar ratio of H3PO4 to Sb as 0.8, 1.0 and 1.3. When the molar ratio is 0.6, 0.8 and 1.3, the average particle size of the colloid with the concentration of 10% are (357±200 )nm, (159±20 )nm and (225±19 )nm, respectively. With increasing amount of H3PO4, the average particle size decreases firstly and then increases. Therefore, when the concentrations of colloidal antimony pentoxide are 10% and 15%, the suitable molar ratios of H3PO4 to Sb range from 0.8 to 1.0 and from 1.0 to 1.3, respectively.
According to the theory of EDL[23] , EDL is a main influence factor of repulsive energy among particles. With increasing amount of H3PO4, both the EDLs thickness of colloidal antimony pentoxide particles and repulsive energy increase, particles are not bound to each other because of collision, and colloidal particles could still keep smaller. With continuous increasing the consumption of the phosphoric acid, the density of electrolyte rises and EDL is compressed, repulsive energy drops instead, and particles size becomes larger because collision causes the small particles to be bound to each other. So the amount of phosphoric acid must be suitable.
It is also be found from Fig.4, that the suitable molar ratio of H3PO4 to Sb is different at different concentrations. The particles counting increases with increasing concentration of Sb2O5, and the distance among them is shortened, both repulsive and attractive energy increase, hence, the stability of antimony pentoxide decreases and particles size becomes larger. With increasing concentration of Sb2O5, increasing the consumption of H3PO4 could enhance the thickness of EDL and repulsive energy, which is an effective way to keep concentrated colloidal antimony pentoxide stable and particles small. Consequently, the suitable molar ratio of H3PO4 to Sb increases with increasing concentration of Sb2O5.
3.3 Effect of mixture concentration on formation and size of colloidal Sb2O5
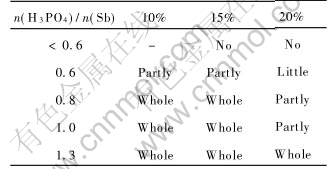
The concentration of colloidal Sb2O5 is dependant upon the concentration of the mixture, the time for forming the colloid, the molar ratio between H3PO4 and Sb for transforming the mixture to colloidal Sb2O5 completely, as listed in Tables 1 and 2.
Table 1 indicates that with increasing in concentration of colloid, the required time for forming the colloid without deposit decreases, which is related to the thermodynamic and kinetic conditions in forming colloid.
The process of forming colloid includes solid
Table 1 Demanded time for forming colloidal Sb2O5 with different concentrations

Table 2 Effect of mixture concentration on formation of colloidal
phase precipitating from liquid phase and growing up, according to the thermodynamics theory that supersaturation is the motive force of this process, the higher supersaturation is, the easier precipitating process is.
Since the concentration of mixture antimony oxide increases, the higher supersaturation of Sb2O5 is reached rapidly and remained, which shorten the reaction time. As a result, the higher concentration of mixture is, the less the reaction time is. Forming colloid includes nucleation and growth. Only the new particles are larger than critical radius, and it can grow up to form stable crystal nucleus without dissolved. Increasing the mixture concentration makes solution keep high supersaturation, and it is profitable to form plenty of crystal nucleuses rapidly. With rising concentration, the nucleation places increase too, which shorten the diffusion time that Sb2O5 molecule spent in diffusing from solution to nucleation place. So incubation time and nucleation time were both shortened. From the view of growth, the speed of growth is accelerated because diffusion distance is shortened. Consequently, both nucleation and growth speed are accelerated, a large number of steady crystal nucleuses form and grow up within shorter time, and reaction time is shorten.
Table 2 demonstrates that with increasing mixture concentration of mixture, the molar ratio of H3PO4 to Sb is increased for transforming all mixture to the colloidal antimony pentoxide, which proves that different concentration of the colloidal antimony pentoxide requires different repulsive energy to remain its stability without flocculation. Because increasing colloidal concentration leads to the increase in particles, the probability of flocculation for the sake of Brownian motion is enhanced. In order to keep colloidal stability, the repulsive energy among particles must increase. To increase the thickness of EDL is an effective way to improve repulsive energy. As a result, the increase in the molar ratio of H3PO4 to Sb, which leads to thicker DEL of colloidal particles and stronger repulsive energy, is the basic reason for the formation and stabilization of colloidal antimony pentoxide.
Fig.5 shows the effect of the concentration of mixture on particles size of colloidal pentoxide. When the molar ratio of H3PO4 to Sb is 1.3, and the concentration of antimony pentoxide is 10%, 15% and 20%, the average diameter of colloidal particles is (225±19)nm, (189±19)nm, and (225±19)nm, respectively. For the 10% colloid solution, the consumption of H3PO4 is so much that EDL of colloidal particles is compressed and the particles diameter grows larger. The consumption is less relatively and EDL is thinner for 20% colloid solution, so diameter of particles is larger too. However, the suitable concentration of the colloidal Sb2O5 with small particle size is 15%.
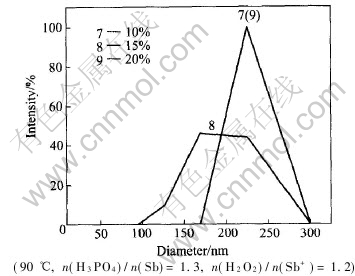
Fig.5 Effect of concentration of mixture on particle size of colloidal Sb2O5
3.4 Effect of temperature on formation and size of colloidal Sb2O5
Fig.6 shows the effect of temperature on size of particles.The average sizes of particles are (163±19)nm, (169±19)nm and (159±20)nm, respectively at the reaction temperature of 60, 75 and 90℃. The results from Fig.6 show no obvious variation of colloidal size. According to the view of kinetics, both nucleation and growth speed are influenced by temperature. With decreasing temperature, molecule kinetic energy decreases while attractive force among molecule is relatively enhanced, and molecule is easy to nucleate and grows up. At the same time, the molecule diffusion becomes difficulty so that speed of nucleation and growth are both slowed down for decreasing temperature. It is clearly that temperature has both advantaged and adverse influence on forming process of colloid and two speeds co-determine the formation of colloid and particles size. However, with the presence of H3PO4, particles are covered by H3PO4 as soon as they precipitate from liquid phase and it could hinder the particles to grow up, so temperature isnt the key factor to affect the forming process of colloid and particles size.
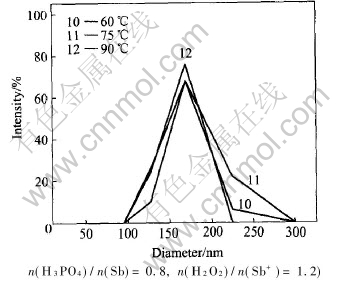
Fig.6 Effect of temperature on particles size of colloidal Sb2O5(90℃, w(Sb2O5)=10%,
4 CONCLUSIONS
1) A novel feasible technology is developed for preparing colloidal antimony pentoxide from arsenic-alkali residue, including water leaching, acid leaching, hydrolyzing and preparing colloidal Sb2O5.
2) Aging of the mixture results in difficulty the formation of colloidal antimony pentoxide, H3PO4 as a stabilizer affects greatly the colloidal particle size. The average particle sizes of the colloid with the concentrations of 10% and 15% are (159±20 )nm and (164±32)nm, respectively at the suitable molar ratios of H3PO4 to Sb ranging from 0.8 to 1.0 and from 1.0 to 1.3.
3) The concentration of colloidal Sb2O5 is dependant upon the concentration of the mixture, the higher the concentration of the mixture is, the longer the time for forming the colloid is, the higher the molar ratio between H3PO4 and Sb for transforming the mixture to the colloidal Sb2O5 is. And the temperature is not the critical factor for preparing colloidal Sb2O5 anymore in existence of H3PO4.
REFERENCES
[1]Crompton C E. Process for Making Colloidal Sols of Antimony Pentoxide in Polyhydroxy Alcohols [P]. US 3994825, 1976.
[2]Crompton C E, Kazi-Abdulla M Z. Process for Making Colloidal Sols of Antimony Pentoxide in Polar Organic Solvents [P]. US 4017418, 1977.
[3]Langere R F, Loeffler D E, Santini F F. Production of Hydrous Pentavalent Antimony Oxide Sol Composition, Dry Powder Prepared Therefrom and Production of Said Dry Powder [P]. US 4026819, 1977.
[4]Gower R P, Richardson J G. Method of Preparing Colloidal Sol of Antimony Oxide [P]. US 4110247, 1978.
[5]Crompton C E, Kazi-Abdulla M Z. Process for Making Colloidal Sols of Antimony Pentoxide in an Aqueous Medium [P]. US 4348301, 1982.
[6]Masaharu S, Shigeo S. Manufacture of Antimony Pentoxide by Oxidation of Antimony Trioxide [P]. JP 8059243, 1996.
[7]WANG Hai-tang, WANG Xiao-wei, ZHANG Wei-yuan, et al. Preparation and stabilization of flame retardant colloidal Sb2O5 using ammonium biphosphate as stabilizer [J]. Fine E Chemicals, 2003, 20(2): 105-108.
[8]TAN Ding-sheng, ZHU Yong-da, ZHU Yong-quan, et al. Size and distribution of colloidal particle in the process of preparing colloid antimony pentoxide with oxidation method [J].Journal of Shanghai University (Natural Science), 1998, 4(6): 698-702.
[9]ZHANG Li, CHEN Wen-mi, GONG Zhu-qing, et al. Size and distribution and its stability of colloidal Sb2O5 particale prepared by refluxing oxidation with hydrogen peroxide as oxidant [J].The Chinese Journal of Nonferrous Metals, 2004, 14(5): 877-882.(in Chinese)
[10]WANG Hai-tang, SHI Qing-ling, WANG Xiao-wei, et al. Optimization of technological preparation condition of colloid Sb2O5 by using sodium borate as stabilizer [J]. Chinese Journal of Applied Chemistry, 2003, 20(5): 496-497.
[11]TAN Ding-sheng, LU Zhi-hua, ZHU Yong-da, et al. Preparation process of colloid Sb2O5 and its stability [J]. Nonferrous Metals, 1999, 51(1): 54-57.(in Chinese)
[12]CHEN Wen-mi, ZHANG Li, GONG Zhu-qing. Preparation of colloidal Sb2O5 and its stability [J]. Trans Nonferrous Met Soc China, 2004, 14(1): 190-193.
[13]ZENG Zheng-ou, ZHAO Rui-rong, GUAN Xuan-guo. Preparing colloidal antimony pentoxide by refluxing oxidation [J]. Hunan Metallurgy, 1990, 3(2): 11-13.(in Chinese)
[14]YAN Jiang-feng. Research on preparing colloidal antimony oxide [J]. Yunnan Meteallurgy, 1995, 4: 33-36.(in Chinese)
[15]WU Jian-Guo. Preparing antimony pentoxide with (NH4)2S2O8 [J]. Inorganic Chemicals Industry, 1991, 1: 9-11.(in Chinese)
[16]Kobashi T, Naka H. Method of Producing Colloidal Antimony Oxide [P]. US 4533538, 1985.
[17]Watanabe Y, Suzuki K, Teranishi M. Process for Preparing Colloidal Solution of Antimony Pentoxide [P]. US 4589997, 1986.
[18]Vogt J W. Method of Preparing Antimony Pentoxide, Colloids Thereof and Their Preparation [P]. US 4351741, 1982.
[19]FENG Duan. Condensed Matter Physics: Metal Physics (Ⅱ). Phase Transformation [M]. Beijing: Science Press, 1998. 128-134.
[20]XU Zu-yao. Principles of Phase Transformation [M]. Beijing: Science Press, 2000. 8-13.
[21]Catone D L. Antimony Pentoxide Dispersions and Method of Making [P]. US 6040371, 2000.
[22]Kobashi T, Naka H. Chemically Stable Colloidal Antimony Oxide [P]. US 4659847, 1987.
[23]ZHENG Zhong. Introduction of Colloid Science [M]. Beijing: Higher Education Press, 1986. 311-319, 16-19, 26-29.
(Edited by LONG Huai-zhong)
Foundation item: Project(2003-07) supported by the Environmental Protection Fund of Hunan Province
Received date: 2005-03-08; Accepted date: 2005-06-10
Correspondence: CHAI Li-yuan, Professor, PhD; Tel: +86-731-8836921; E-mail: lychai@mail.csu.edu.cn