Deoxidation of H13 tool steel with CaF2-MgO-CaO-Al2O3-SiO2 slags at 1873 K
来源期刊:中南大学学报(英文版)2021年第2期
论文作者:郭汉杰 李少英 李彬 段生朝 赵兴明 郭靖
文章页码:370 - 385
Key words:deoxidation; H13 tool steel; slag-steel interface reaction; slag optimization
Abstract: Laboratory-scale experiments were performed to investigate the deoxidation of H13 tool steel with CaF2-MgO-Al2O3-CaO-SiO2 slags at 1873 K. The calculation of thermodynamics and kinetics was also verified through the experimental results. The results show that [Si]-[O] reaction is the control reaction, and with the increase of basicity of slag, the limitation of deoxidation was decreased. The limitation of deoxidation is the lowest for the slag with basicity of 2.0. Under the conditions of the basicity of 2.0 and the content of CaF2 more than 50%, the limitation of deoxidation is less than 10×10-6, and it does not depend on the contents of Al2O3 and CaF2 in slags. The mass transport of oxygen in the metal phase is the rate-controlling step, and the slag composition has no effect on the equilibrium time of deoxidation. Based on this finding, the optimized slag composition is designed and it contains the following components: 51.5% CaF2, 20.3% MgO, 16.2% Al2O3, 8.2% CaO and 3.8% SiO2. In the case of the optimized deoxidizing slag, the total oxygen content in H13 steel can be reduced from 25×10-6 to 6×10-6.
Cite this article as: LI Shao-ying, LI Bin, DUAN Sheng-chao, ZHAO Xing-ming, GUO Jing, GUO Han-jie. Deoxidation of H13 tool steel with CaF2-MgO-CaO-Al2O3-SiO2 slags at 1873 K [J]. Journal of Central South University, 2021, 28(1): 370-385. DOI: https://doi.org/10.1007/s11771-021-4609-x.

J. Cent. South Univ. (2021) 28: 370-385
DOI: https://doi.org/10.1007/s11771-021-4609-x

LI Shao-ying(李少英)1, 2, LI Bin(李彬)1, 2, DUAN Sheng-chao(段生朝)1, 2,
ZHAO Xing-ming(赵兴明)3, GUO Jing(郭靖)1, 2, GUO Han-jie(郭汉杰)1, 2
1. School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, Beijing 100083, China;
2. Beijing Key Laboratory of Special Melting and Preparation of High-end Metal Materials,Beijing 100083, China;
3. Shandong Tianwu Forming Technology Co., Ltd., Liaocheng 252024, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract: Laboratory-scale experiments were performed to investigate the deoxidation of H13 tool steel with CaF2-MgO-Al2O3-CaO-SiO2 slags at 1873 K. The calculation of thermodynamics and kinetics was also verified through the experimental results. The results show that [Si]-[O] reaction is the control reaction, and with the increase of basicity of slag, the limitation of deoxidation was decreased. The limitation of deoxidation is the lowest for the slag with basicity of 2.0. Under the conditions of the basicity of 2.0 and the content of CaF2 more than 50%, the limitation of deoxidation is less than 10×10-6, and it does not depend on the contents of Al2O3 and CaF2 in slags. The mass transport of oxygen in the metal phase is the rate-controlling step, and the slag composition has no effect on the equilibrium time of deoxidation. Based on this finding, the optimized slag composition is designed and it contains the following components: 51.5% CaF2, 20.3% MgO, 16.2% Al2O3, 8.2% CaO and 3.8% SiO2. In the case of the optimized deoxidizing slag, the total oxygen content in H13 steel can be reduced from 25×10-6 to 6×10-6.
Key words: deoxidation; H13 tool steel; slag-steel interface reaction; slag optimization
Cite this article as: LI Shao-ying, LI Bin, DUAN Sheng-chao, ZHAO Xing-ming, GUO Jing, GUO Han-jie. Deoxidation of H13 tool steel with CaF2-MgO-CaO-Al2O3-SiO2 slags at 1873 K [J]. Journal of Central South University, 2021, 28(1): 370-385. DOI: https://doi.org/10.1007/s11771-021-4609-x.
1 Introduction
The detriments of non-metallic inclusions to the performance of steel have become a consensus among researchers [1-3]. Reducing the oxygen content in the steel can minimize the amount of inclusions, thereby improving the performance of the steel [4]. For example, when the total content of oxygen (i.e., [O]T) of bearing steel is reduced from 15×10-6 to 5×10-6, the fatigue life can be increased by 100 times [5]; also, when [O]T of carburized gear steel is reduced from 25 ×10-6 to 11×10-6, the fatigue life can be increased by 4 times [6]. With the decrease of [O]T in the tool steel, the hardness that begins to crack increases. In the common hardness of die steel (48-50 HRC), [O]T is controlled below 15×10-6 to reduce the possibility of cracking [7]. However, when the hardness is higher than HRC 50 (such as shield machine tools), [O]T in the steel is required to be less than 10×10-6 [8]. Therefore, it is necessary to produce ultra-low oxygen die steel.
Since electroslag remelting (ESR) generally decreases oxygen content and significantly removes non-metallic inclusions in steel, it has become the final step of liquid steel refining for producing high-end die steel [9, 10]. In the refining process, oxygen in the air is one of the main sources of oxygen in electroslag ingots, which can be transferred to the molten metal pool by oxidizing the electrode surface before melting and directly penetrating through the slag layer [11]. To obtain the lower oxygen potential of the molten slag, the argon protection and the slag deoxidation treatment are conducted during the electroslag remelting process. CHEN et al [12] proposed that during the ESR process of Inconel 718 alloy under Ar atmosphere, the mechanical mixture of 20 wt% Al, 20 wt% Al2O3, 20 wt% CaF2, and 40 wt% iron powder was used as the deoxidant of slag. When the oxygen content of the electrode is 7×10-6, the oxygen content of ingot is increased to 8×10-6 with slag deoxidation treatment, while the oxygen content ingot is increased to 16×10-6 without slag deoxidation treatment. SHI et al [10] also applied the same slag deoxidation treatment to the electroslag remelting of S136 die steel in the 50 kg ESR furnace. Under the Ar atmosphere, the oxygen content can be reduced from 89×10-6 in the electrode to 12×10-6 in the ingot.
The essence of deoxidation of electroslag remelting is the deoxidation by the slag-steel interface reaction [13]. In this process, the oxygen content of liquid steel is determined by the interactions of atmosphere-slag-metal-inclusion. Therefore, in addition to deoxidizing agents and remelting atmosphere, deoxidation is affected by alloy compositions, slag compositions, absorption ability of slag to oxide inclusions, and oxide inclusion evolution [3]. Among these factors, slag compositions play a major role in the deoxidation process of the specific steel. Several efforts have been made to investigate the effect of slag compositions on the deoxidation by the slag-steel interface reaction. DUAN et al [13] described the deoxidation behavior in the reactions between slag and metal phases by the simultaneous equilibrium of the interfacial reaction (CaO)-(Al2O3)-(MgO)- (TiO2)-[S]-[O], and concluded that [O]T in Inconel 718 superalloy decreased with an increase of CaO, MgO and CaF2 content in the slag, and CaO had a great influence on the deoxidation compared with MgO and CaF2 in the slag. They found that when the slag contained the following components of 45.02 wt% CaF2, 24.05 wt% CaO, 24.05 wt% Al2O3, 2.86 wt% MgO and 2.86 wt% TiO2, [O]T in steel decreased to 13×10-6 from 33×10-6 in the consumable electrode. At the slag-steel interface of high-frequency induction furnace, PARK et al [14] also found the similar phenomenon that [O]T in Fe-0.2%O alloy was decreased with increasing basicity, and the content of MgO and CaF2, whereas it was increased by increasing the content of Al2O3 in the slag. They found that the lowest oxygen content in Fe-O alloy is not less than 20×10-6. During the process of electroslag remelting in CrNiMo low alloy steels, [O]T of the ingots decreased with the decrease of Al2O3 activity in the slag pool with the constant content of Al in the electrode, and the lowest oxygen content in steel is 34×10-6 [15].
Previous researches showed that in the slag-steel interface reaction, the influence of each component in slag on the deoxidation of different steel grades can be not identical [13-15]. Nevertheless, few experiments have been done to understand the deoxidation of H13 tool steel using CaF2-MgO-CaO-Al2O3-SiO2 slag. In the current study, the effect of CaO/SiO2, CaF2, and Al2O3 in CaF2-MgO-CaO-Al2O3-SiO2 on the limitation of deoxidation in H13 tool steel was investigated using laboratory-scale experiments and thermodynamic calculation. Furthermore, the deoxidation mechanisms were also discussed, respectively, based on experimental results and the prediction results of kinetics. This study aims to provide tool steel of ultra-low oxygen control technique which can be effectively applied to industrial refining operation.
2 Experimental
2.1 Materials preparations
Before the experiment, H13 tool steel samples were produced in a vacuum-induction melting furnace under Ar atmosphere, and the chemical composition of ingots is shown in Table 1. The steel block of smaller size (1 to 1.5 cm3) was cut from the ingot to facilitate weighing. Iron oxide on the surface of steel blocks was removed by sandpaper. Pre-melted slags were prepared with analytical reagent powders (Sinopharm Chemical Reagent Co., Ltd.) of CaO, SiO2, MgO, Al2O3, and CaF2. The composition of the slag used in the study is listed in Table 2. The effect of basicity on oxygen content can be compared by Slag-1, Slag-2 and Slag-3. The effect of Al2O3 content on oxygen content was compared with Slag-3, Slag-4 and Slag-5. The effect of CaF2 content on oxygen content was compared with Slag-4, Slag-6 and Slag-7. In order to prevent the erosion of MgO crucible, the content of MgO in the slag was set as saturated solubility.
Table 1 Chemical composition of molten metal used in this study (mass fraction, %)

Table 2 Chemical composition of slags used in this study

2.2 Experimental equipment and procedures
Deoxidation experiments were performed in a vertical-type tube furnace (Braveman, Luoyang Braveman Special Testing Furnace Co., Ltd.) equipped with an alumina reaction tube (inner diameter of 80 mm, outer diameter of 90 mm, and height of 1000 mm). To prevent the oxygen in the air from entering the furnace tube, the whole furnace tube was filled with high purity argon, so that the refractory lid on the top of the furnace tube was slightly opened. The temperature was controlled by a PID controller connected to a double Pt-Rh thermocouple of type B. There was a double-layer graphite crucible with built-in MgO crucible [16].
The molten steel was placed in the lower layer of MgO crucible and the outer layer of MgO was covered with graphite crucible. The slag was placed in the upper layer of graphite crucible. The graphite plug in the upper layer can control the reaction time of slag and steel. The schematic of the experimental apparatus is shown in Figure 1, and the operation steps in the smelting experiments are illustrated in Figure 2.
1) The slag was prepared in a graphite crucible (outer diameter of 50 mm, inner diameter of 44 mm, height of 88 mm), and then graphite crucible was placed in an electric resistance furnace under purified Ar atmosphere, which was positioned in the constant-temperature zone from the room temperature to 1873 K and kept 20 min at 1873 K to ensure the uniform composition.

Figure 1 Schematic of experimental equipment used in this study

Figure 2 Schematic of operation steps in smelting experiments
2) The pre-melted slag (13 g) was held in the upper graphite crucible with a small hole in its bottom. To ensure that the melted slag drops fully into the lower crucible, the inner wall angle of upper crucible is 110°. The graphite plug at hole was used to control the reaction time of slag and steel. After polishing surface, the test steel (46 g) was prepared in MgO crucible (outer diameter of 30 mm, inner diameter of 25 mm, height of 35 mm). The outer layer of MgO crucible was covered with graphite crucible (outer diameter of 36 mm, Inner diameter of 31 mm, height of 40 mm) to protect from rupture of MgO crucible. For ease of operation, the big graphite crucible (outer diameter of 66 mm, inner diameter of 54 mm, height of 140 mm) was placed on the outside of the double layer crucible.
3) After temperature reached 1873 K, the double layer crucible was placed in electric resistance furnace in purified Ar atmosphere (2-4 L/min). Meanwhile, the temperature was decreased. When the temperature was recovered to 1873 K, the steel and slag were kept for 15 min to ensure that they were completely melted. At the moment, the graphite plug was removed. The reaction between slag and steel began. Namely, the moment was taken as the starting time for the reaction.
4) After certain reaction time intervals (1, 3, 6, 10 and 30 min), the whole crucible was rapidly removed from furnace and placed in bucket with ice water. After the completion of the reaction between slag and steel, each sample of about 1 g was removed from these reaction products to analyze the content of total oxygen by oxygen and nitrogen hydrogen analyzer (TCH600, LECO, USA). At least three samples of metal were taken to analyze the composition at each test run, and the average of the three results was used as the oxygen content in the ingot.
3 Results and discussion
3.1 Variation of oxygen content in steel
In this study, the effects of CaO/SiO2, CaF2 and Al2O3 on the variation of oxygen content in the H13 tool steel at 1873 K were investigated. The total oxygen contents in the experimental ingots were measured, as shown in Table 3. The oxygen content considerably deceased from 25×10-6 to a nearly constant value (6×10-6 to 16×10-6) within the reaction time of 10 min, and the oxygen content at 10 min was almost equal to that at 30 min. It can be inferred that no reoxidation of liquid steel occurred in laboratory-scale experiments. In addition, the FeO content in the final slag does not exceed 0.05% analyzed by X-ray fluorescence, which illustrates that in the case of laboratory-scale experiment under Ar gas protective atmosphere, the presence of FeO in molten slag can be prevented.
The final oxygen content decreases to 6.3×10-6 from 16.4×10-6 with increasing binary basicity from 0.2 to 2.0 in CaF2-MgO-CaO-Al2O3-SiO2 slag. It can be inferred that the binary basicity of slag has influence on the oxygen content in the steel. The changes of Al2O3 and CaF2 content in slag with the basicity of 2.0 were found to make a negligible difference in the oxygen content in H13 steel. The decrease in the oxygen content can be explained by the Si-deoxidation at the slag-steel reaction.
3.2 Thermodynamic on oxygen variation in steel
According to the standard Gibbs free energy of the reaction (i.e., △GΘ), it can be found that the deoxidizing ability of Al element in steel is evidently stronger than that of Si element, as shown in Figure 3. However, in the slag-steel interface reaction, the activity of the reaction products and reactants is not in the standard state, which has an effect on the deoxidization ability of the elements. Therefore, the Gibbs free energy of the reaction (△G) can be used to judge the deoxidization ability of Si and Al, as shown in Eqs. (1) and (2) [17].
Table 3 Total oxygen content of experimental ingots

1/2[Si]+[O]=1/2SiO2,
△G1Θ=-290520+110.8T (1)
2/3[Al]+[O]=1/3Al2O3,
△G2Θ=-406266.3+131.376T (2)
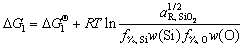 (3)
(3)
 (4)
(4)
In the calculation process, the relevant parameters are required, which include the activity of component i(aR,i) in slag, the activity coefficients (f%,i) of Si, Al and O, the reaction temperature, and the chemical composition of H13 tool steel listed in Table 1 (w(Si), w(O), w(Al)). The calculation of aR,i can be conducted by the activity model based on the ion and molecule coexistence theory, and the details of the modelling and relevant parameters were shown in previous studies [18, 19]. Since the concentration of iron in H13 steel is greater than 90 wt.%, the primary interaction coefficients (i.e., 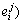 , which are listed in Table 4, can be used to calculate the activity coefficient (f%,i) of component i in steel based on the Wagner equation [17]. In general, the temperature range during steelmaking process is 1823 K to 2023 K, and the temperature of 1873 K was selected in this study.
, which are listed in Table 4, can be used to calculate the activity coefficient (f%,i) of component i in steel based on the Wagner equation [17]. In general, the temperature range during steelmaking process is 1823 K to 2023 K, and the temperature of 1873 K was selected in this study.

Figure 3 Relationship between △GΘ and T with different slag compositions (Solid line: [Si]-[O]-(SiO2); dotted line: [Al]-[O]-(Al2O3); black: Slag-1; blue: Slag-2; red: Slag-3; brown: Slag-4; orange: Slag-5; pink: Slag-6; green: Slag-7)
The relationship between △GΘ and T with different slag compositions is plotted in Figure 3. Compared with the difference value of △GΘ between [Si]-[O] reaction and [Al]-[O] reaction, the difference value of △G between [Si]-[O] reaction and [Al]-[O] reaction is smaller. In the temperature range of 1750-1950 K, the lines of [Al]-[O] and [Si]-[O] almost coincide under the conditions of Slag-2 to Slag-7, indicating that the binding capacity of [Al] and [O] is basically similar to that of [Si] and [O].
The dissolved oxygen content (i.e., the limitation of deoxidation) is related to the [Si]-[O] equilibrium and the [Al]-[O] equilibrium. The oxygen content of liquid steel in equilibrium with SiO2 in the slag can be expressed as [O]Si. The oxygen content equilibrated with dissolved aluminum (0.02 wt%) in liquid steel and Al2O3 in the slag can be expressed as w(O)Al. The relationship between the [O]Si and [O]Al in H13 tool steel and the content of each component in CaF2-CaO-Al2O3-MgO-SiO2 slags can be obtained by mathematical derivation, as shown in Eqs. (5) and (6).

 (5)
(5)
Table 4 Interaction coefficients used in the present study [17]
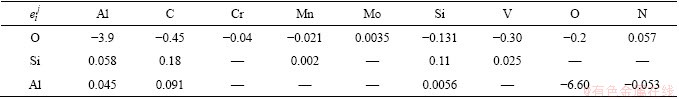

 (6)
(6)
The relationships between the [O]Si and [O]Al of H13 tool steel and the slag composition of component i in CaF2-MgO-CaO-Al2O3-SiO2 slag at the temperature of 1873 K are illustrated in Figures 4-7.
3.2.1 Determination of MgO content in CaF2- MgO-Al2O3-CaO-SiO2 slag
MgO, as a basic compound, may affect the deoxidation of H13 tool steel. Therefore, for slags with basicity of 0.2 and 2.0, the effects of MgO content in slag (w(MgO), the same as below) on the oxygen content in steel were analyzed, respectively. Figure 4(a) shows the dependence of [O]Si on MgO content in slag with basicity of 0.2 and 2.0 (w(MgO)+w(CaF2)=72%,w(CaO)+w(SiO2)=12% and w(Al2O3)=16%). When the basicity is 0.2, with the increasing addition of MgO, the equilibrium [O]Si content decreases continuously, and the equilibrium [O]Si content is less than 10×10-6 as the MgO content increased above 28%; while when the basicity is 2.0, with the increase of w(MgO), the equilibrium [O]Si content does not decrease significantly, and when the MgO content in slag is arbitrary, the equilibrium [O]Si content in steel is less than 10×10-6, indicating that the w(MgO) in high basicity slag has no influence on deoxidation. The results can be explained in Figure 4(b). When the basicity is 0.2, the activity of SiO2 decreases as w(MgO) increases, whereas, when the basicity is 2.0, the activity of SiO2 has no change with the increase of w(MgO). According to the order of the optical basicity of the oxide in slag (i.e.,  [17], it can be inferred that the ability of MgO combined with SiO2 is lower than the ability of CaO combined with SiO2, and this leads to no change in the activity of SiO2 with the increase of w(MgO) at the basicity of 2.0. Figure 4(b) illustrates quantitatively the above phenomenon.
[17], it can be inferred that the ability of MgO combined with SiO2 is lower than the ability of CaO combined with SiO2, and this leads to no change in the activity of SiO2 with the increase of w(MgO) at the basicity of 2.0. Figure 4(b) illustrates quantitatively the above phenomenon.

Figure 4 Relationship between w(MgO) and oxygen content in steel (a) and aAl2O3 and aSiO2 in slag (b); Relationship between MgO saturated solubility and w(CaO)/w(SiO2) at w(Al2O3)=16% (c) and w(Al2O3) at w(CaO)/w(SiO2)=2.0 (d)

Figure 5 Relationship between w(CaO)/w(SiO2) and oxygen content in steel (a), aAl2O3 and aSiO2 in slag (b) with w(MgO)=20% and w(CaO):w(Al2O3)=1:1

Figure 6 Relationship between w(Al2O3) and oxygen content in steel (a), aAl2O3 and aSiO2 in slag (b) with w(MgO)=20% and w(CaO):w(Al2O3)=1:1

Figure 7 Relationship between w(CaF2) and oxygen content in steel (a), aAl2O3 and aSiO2 in slag (b) and contents of CaF2 in slags (w(CaO)/w(SiO2)=2.0 and w(CaO):w(Al2O3)=1:1
Although the activity of Al2O3 decreases continuously with increasing the w(MgO), the change of the equilibrium [O]Al content can be ignored, as shown in Figures 4(a) and (b). This will be explained in detail in Section 3.2.3. In a word, when the basicity is 0.2, the increase of MgO content is beneficial to reduce limitation of deoxidation, but when the basicity is 2.0, the change of MgO content has no effect on the limitation of deoxidation.
During the steelmaking process, the MgO content in slag must reach saturation solubility to avoid erosion of the MgO crucible [20]. The relationship between the basicity or w(Al2O3) and the saturation solubility of MgO in slag was calculated by Equilib module and FToxid database in Factsage 7.0, and the results are shown in Figures 4(c) and (d). According to the results, the range of saturation solubility of MgO in slag is 14.5% to 20.5% for slag with basicity of 0.2 to 2.0 and Al2O3 content of 4% to 16%. In order to avoid the corrosion of MgO crucible, the MgO content in slag system is determined to be 20%.
3.2.2 Effects of w(CaO)/w(SiO2) on limitation of deoxidation
In Figure 5(a), the calculated [O]Si and [O]Al in the molten metal are plotted against the slag basicity in slag (w(MgO)=20%, w(CaO)+[SiO2]= 12%, w(Al2O3)=w(CaO)). The equilibrium [O]Si decreases sharply under the condition of w(CaO)/w(SiO2)<2.13, and the point of intersection of the [O]Si line and the [O]Al line is 4.65×10-6 at w(CaO)/w(SiO2)=2.13. When the basicity is greater than 2.13, basicity has little effect on [O]Si. This can be contributed to the variation of  in Figure 5(b). In addition, the equilibrium [O]Al is always less than 5×10-6 over the full basicity range, which will be further explained in Section 3.2.3.
in Figure 5(b). In addition, the equilibrium [O]Al is always less than 5×10-6 over the full basicity range, which will be further explained in Section 3.2.3.
Except for the predicted values of the limitation of deoxidation, Figure 5(a) also shows the experimental results for the total oxygen content in steel with basicity for the three slag compositions at 1873 K. It can be seen from Figure 5(a) that the measured total oxygen at the reaction time of 30 min, which includes both the dissolved oxygen (free oxygen) and the oxygen bonded as oxide inclusions, in the experimental ingot is between the limitation of deoxidation determined by [Al]-[O] equilibrium and by [Si]-[O] equilibrium, especially for the basicity less than 2.13. When the basicity is more than 2.13, the dissolved oxygen content determined by [Si]-[O] equilibrium is almost equal to that by [Al]-[O] equilibrium. The total oxygen content with the basicity of 2.13 is slightly higher than the limitation of deoxidation, for which the total oxygen content is lower than the dissolved oxygen in liquid steel. Combined with the experimental results, it can be found that the limitation of deoxidation is determined by [Si]-[O] equilibrium. This is consistent with the result of HOU et al [21], who reported that increasing the basicity in CaF2-CaO-Al2O3-MgO-TiO2-SiO2 slag decreased the oxygen content of 1Cr21Ni5Ti steel by reducing the activity coefficient of SiO2. Moreover, PARK et al [14] also found that the high basicity slag is conducive to the decrease in [O]T of Fe-0.2 wt% O alloy.
3.2.3 Effects of Al2O3 content in slag on limitation of deoxidation
When the content of Al2O3 in the slag (w(MgO)=20%, w(CaO)=8%, w(SiO2)=4%, w(Al2O3)+w(CaF2)=68%) is 4
When the slag-steel reaction reached equilibrium (i.e., the reaction time is 30 min), the total oxygen content of the three laboratory-scale experimental ingots at 1873 K under various Al2O3 contents is shown by the gray balls in Figure 6(a). With the increase of Al2O3 content, the total oxygen content in liquid steel almost unchanged, which is consistent with the calculated results. It can be inferred that it is difficult to reduce the limitation of deoxidation for H13 tool steel only by adjusting the mass content of Al2O3 in slag.
3.2.4 Effects of CaF2 content in slag on limitation of deoxidation
As shown in Figure 7, the addition of CaF2 to slag (w(MgO)=20%, w(CaO):w(Al2O3):w(SiO2)= 2:2:1) does not significantly change the [O]Al and [O]Si in steel. In the electroslag refining process, since CaF2 plays an important role in improving the fluidity and conductivity of the slag, the content of CaF2 in the slag will have a certain effect on the flotation of inclusions, which affects the total oxygen content of the molten metal. Figure 7(a) also presents comparisons between the calculated and measured oxygen content reaching the reaction equilibrium under various CaF2 contents in CaF2-CaO-Al2O3-MgO-SiO2 slag at 1873 K, showing that the oxygen content depends weakly on the CaF2 content in the studied slags and the changing trend is consistent with the simulation results. It should be pointed out that the measured oxygen content includes dissolved oxygen in steel and oxygen in inclusions. Although the removal rate of inclusions increased with increasing CaF2 content in CaF2-CaO-Al2O3-MgO-SiO2 slag at 1873 K [22], when the CaF2 content in slag exceeds 20%, the fluoride evaporates from the slag, causing the remaining fluoride to have no significant influence on the change of the inclusion removal [23]. In this study, the content of CaF2 in the experimental slag system exceeds 50%, so the addition of CaF2 has no influence on the removal of inclusions, and the measured oxygen content does not decrease with the increase of CaF2.
The equilibrium of the slag-steel reaction means that the [Al]-[O] reaction and the [Si]-[O] reaction reach equilibrium at the same time, that is, the values of [O]Si and [O]Al are equal. According to the calculation results, it can be found that when the basicity of slag is 2.0, as the slag composition changes, the values of [O]Si and [O]Al remain unchanged and are basically equal. This means that under this condition, the limitation of deoxidation value is determined by the [Si]-[O] reaction or the [Al]-[O] reaction. [O]Si decreases and [O]Al remains basically unchanged as the basicity increased up to 2.0. Under the condition, the limitation of deoxidation should be between [O]Si and [O]Al. Combined with the experimental results, it can be found that the change trend of total oxygen content with basicity is consistent with the change trend of [O]Si content with basicity, which means that the deoxidation limit value is determined by [Si]-[O]. In addition, it can be inferred that the effect of w(CaO)/w(SiO2) on controlling the limitation of deoxidation in H13 tool steel compared with CaF2, Al2O3, and MgO in CaF2-MgO-CaO-Al2O3-SiO2 slags is very severe.
3.3 Kinetic analysis
3.3.1 Development of mass transfer model
According to the analysis of thermodynamics, the [Si]-[O] reaction is the control reaction. To investigate the effect of slag composition on deoxidation reaction rate, the mass transfer models of [Si]-[O] reaction can be established. The description is as follows.
According to the reaction mechanism between molten steel and slag, reaction (1) can be divided into four steps. Since the interface chemical reaction is sufficiently rapid, the overall reaction rate is controlled by mass transfer of an element (or compound) to by the slag-metal interface through the concentration boundary layer [13].
In the part, the metal-only control model and the mixed (metal and slag) control model are discussed. The flux of component i across unit area, Ji, is defined in Eq. (7). For the reaction controlled by one species, a composition change with time can be expressed in Eq. (8). Equations (7) and (8) can be connected by the equal difference between the bulk’s concentration and the concentration at interface. Surely, concentration unit should be converted by projected interface area, the mass of solvent and the density of solvent.
 (7)
(7)
 (8)
(8)
where Ji represents the element flow across the slag-metal interface, mol/(m2·s); ki represents the mass transfer coefficient of component i, m/s; Cb is bulk’s concentration and Ci is interfacial concentration; A is projected interface area, m2; m is the mass of solvent, and ρ is density of solvent.
(1) Metal-only control model
In the metal-only control model, the concentrations of reactants (i.e., [Si] and [O]) change continuously on the metal boundary layer, while the slag has no boundary layer. In other words, there is no concentration gradient in slag. This model includes the rate of reaction controlled by mass transport of O in liquid steel and by mass transport of O and Si in liquid steel. The composition changes of reactants and product in reaction (1) can be obtained by Eq. (7) with the assumptions [22]: 1) The reactions reach chemical equilibrium state quickly at the metal-slag interface. 2) The reactants and product have no accumulation at interface, and their concentrations are evenly distributed.
If the rate of reaction is controlled by mass transport of O in liquid steel, there are four unknowns in these equations: w(Si)b=w(Si)i, w(SiO2)b=w(SiO2)i, w(O)b and w(O)i. If the rate of reaction is controlled by mass transport of O and Si in liquid steel (w(Si)b≠w(Si)i), there are five unknowns. These unknowns can be solved by combining mass transfer equations, mass balance equations, and chemical equilibrium equations.
Mass transfer equations: Equation (9) can be obtained by Eq. (7):
 (9)
(9)
where nO presents amount-of-substance of oxygen, mol; cO,i is the molarity of oxygen at the metal-slag interface, mol/L; cO,b is the molarity of oxygen in the metal, mol/L.
 (10)
(10)
 (11)
(11)
where Vmetal is the volume of metal, L.
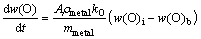 (12)
(12)
 (13)
(13)
Mass balance equations:
 (14)
(14)
 (15)
(15)
According to the assumption, there is no accumulation on the interface,
 (16)
(16)
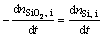 (17)
(17)
 (18)
(18)

 (19)
(19)
Chemical equilibrium equations:
 (20)
(20)
where K is an apparent equilibrium constant. Due to w(SiO2)i= w(SiO2)b,


 (21)
(21)
If the reaction rate controlled by mass transport of O in liquid steel, K can be expressed by Eq. (22).

 (22)
(22)
In the case of the reaction rate controlled by mass transport of O and Si in liquid steel, the relationship between the component’s concentration and time can be obtained by considering the formulas (12), (13), (16), (19) and (21). For the reaction rate controlled by mass transport of O in liquid steel, the unknowns are solved by simultaneous Eqs. (12), (16), (19) and (22).
(2) Mixed control model of metal and slag mass transfer
When the mass transports in both metal and slag phases are considered, Eq. (13) and (14) should be modified as follows. Since mass transport of SiO2 is no longer high, fluxes and mass balances must be taken into account separately. Hence, there should be six unknowns: w(Si)b, w(O)b, w(SiO2)b, w(SiO2)i, w(Si)i, and w(O)i.
Mass transfer equations:
 (23)
(23)
 (24)
(24)
 (25)
(25)
Mass balance equations:
 (26)
(26)
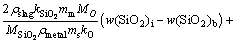
 (27)
(27)

 (28)
(28)
Chemical equilibrium equation:


 (29)
(29)
The chemical equilibrium equation at this model is different from metal-only control model. This difference is attributed to w(SiO2)i ≠w(SiO2)b. Equations can be solved as differential-algebraic equations by Matlab.
(3) Determination of mass transfer coefficient
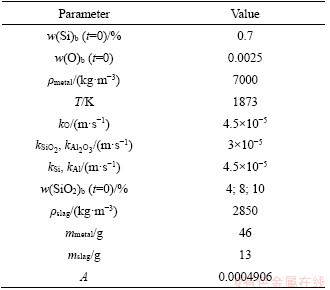
The parameters in mass transfer models are listed in Table 5. The equilibrium content of each component is obtained by thermodynamics. The density of metal and slag can be determined by a previous study [24], and the reaction area is calculated by the diameter of reaction crucible. In addition, the effect of mass transfer coefficient on deoxidation rate is also discussed.
Several reports showed that mass transfer coefficient of a certain component is not a definite value under different reaction conditions. For instance, during electroslag remelting process, the value of kO is 9.1×10-5 m/s for the [Al]-[O] reaction in G20CrNi2Mo bearing steel [25]; while the value of kO is 6×10-4 m/s for the [Al]-[O] reaction in 99.96%Fe-0.04%Al alloys [26]. Since the actual value of mass transfer coefficient varies with experimental conditions, such as heating condition and container dimensions, these conditions were assumed as follows: 1) The value of kSi is equal to the value of kO; 2) The value of kSiO2 is equal to 3×10-5 m/s.
Table 5 Initial parameters required for kinetic model calculation
Under these assumptions, the effect of kO on the reaction rate is discussed. It’s worth noting that these preconditions were only applied to discussing the value of kO. Figure 8(a) shows the effect of different kO values on the reaction rate in [Si]-[O] reaction for metal-only control model. With the increase of kO, the rate of reaction increases. When kO is varied from 9×10-6 m/s to 9×10-4 m/s, the different prediction values of the oxygen are compared with the total oxygen measured values at different sampling time in the experiment of Slag-3. It can be inferred that when the value of kO is 4.5×10-5 m/s, the rate curve is consistent with the experiment results.
In order to ensure the reasonable mass transfer coefficient, the effect of the ratio of kO to kSi (or kO to kSiO2) on the reaction rate is discussed in metal-only and mixed models, respectively. In this discussion, the metal-only model refers to the rate of reaction controlled by mass transport of O and Si in liquid steel. Suppose that assumption 2) holds and the value of kO is 4.5×10-5 m/s. When the value of kSi changes from 0.01 time kO (abbreviated as 0.01kO, and the value is 0.014.5×10-7 m/s) to 100kO (4.5×10-3 m/s), the reaction rates are compared in Figure 8(b) for the metal-only control model of [Si]-[O] reaction. From the results of the analysis, when the value of kO/kSi increases from 0.01 to 100, the reaction rates are indistinguishable; hence, the definition of kO/kSi=1 is reasonable in the metal-only control model of [Si]-[O] reaction.

Figure 8 Effect of different mass transfer coefficients on changes of w(O)Si with time:
Since the mass transfer coefficient of SiO2 (kSiO2) decreases with increasing the viscosity of slag, the mass transfer coefficient of slag is dependent on the slag composition in the mixed control model [26]. Therefore, it is necessary to consider the effect of kSiO2 on reaction rate in mixed control model.
To simplify the analysis, kSiO2 is substituted to kO/kSiO2 in the calculation. The results show that the difference of kO/kSiO2 has no significant effect on the reaction rate in Figure 8(c). According to this, the value of kSiO2 was defined as 3×10-5 m/s in Table 5. This result is consistent with the mass transfer coefficient obtained by solute penetration theory [25].
3.3.2 Validity of developed mass transfer model
The validity of developed mass transfer model is verified by checking the experimental results and the balanced compositions acquired by thermodynamics. The parameters involved in the calculation of mass transfer model are listed in Table 5. The effect of slag composition on deoxidation rate will be discussed below. In the section, the total oxygen content in the test steel is compared with the oxygen content predicted by mass transfer model, which facilitates the detailed explanation of the relationship between slag composition and the deoxidation rate of H13 die steel. Figure 9 shows the changes of the oxygen content in metal phase calculated by two rate-controlling models and total oxygen measured values with reaction time for the various slag compositions at 1873 K. The solid and dashed lines represent w(O)Si of bulk and w(O)Si of interface respectively.
Kinetic calculation results show that the rate of reaction in the mixed control case is the same as that in the metal-only control case which includes the mass transport of [Si] and the mass transport of [Si] and [O], as shown by the solid lines in Figure 9. It can be inferred that under the conditions of the present study, the rate-controlling step is the mass transport of oxygen in the metal phase rather than the mass transport of SiO2 in the slag phase (and the mass transport of Si in the metal phase) for the kinetic model of deoxidation. This result arises from the large value of apparent equilibrium constant, which can be seen in the chemical equilibrium Eqs. (21), (22) and (29). As discussed in Section 3.3.1, when the values of kSi and kSiO2 changes, the difference in the reaction rate is insignificant in both metal-only control model and mixed control model.

Figure 9 Oxygen content in H13 tool steel as a function of reaction time for various slags at 1873 K:
To validate the developed mass transfer model, the results of the three laboratory-scale experiments about the slag-1, slag-2 and slag-3 at 1873 K are also shown in Figure 9(a), which indicates that the changing trend is consistent with the simulation results. It is demonstrated that the rate of deoxidation in this study is determined by [Si]-[O] reaction. With the increase of basicity, the total oxygen content decreases, but the equilibrium time of deoxidation reaction for H13 tool steel is about 10 min. The constant relationship between the rate of deoxidation and slag basicity was reported by DUAN et al [13] in the study of CaF2-CaO-Al2O3-MgO-TiO2 slag at 1873 K.
Figure 9(b) compares the calculated oxygen content with the measured value of total oxygen under different Al2O3 contents in CaF2-CaO-Al2O3- MgO-SiO2 slag at 1873 K. The results indicate that the dependence of oxygen content in steel on Al2O3 content in the studied slag is weak, and the measure total oxygen content are consistent with the prediction results. If the inclusion is Al2O3-rich, the removal rate of inclusion is strongly affected by the solubility of Al2O3 in the slag phase. In the present study, the [Si]-[O] reaction is the controlling reaction, and the inclusions in steel is SiO2-rich rather than Al2O3-rich. Consequently, there is no evident variation of the T[O] caused by the change of Al2O3 content.
According to the analysis of thermodynamics, the content of [O]Si decreases with the increase of CaF2 content. When the content of CaF2 exceeds 50%, the content of [O]Si reduces slightly. The effect of CaF2 content on deoxidation rate in H13 tool steel is shown in Figure 9(c). The total oxygen content is greater than the predicted results, but the changing trend of calculation results is similar to the experimental data. When the content of CaF2 in slag increased from 50% to 70%, the range of [O]T is 6-9×10-6, and it illustrates that the effect of CaF2 content on the deoxidation rate is negligible.
The calculation results and experimental results show that there is small difference between the experimental data and the predicted value, and the difference value is oxygen in the form of oxide inclusions. The predicted results show that the effect of slag composition on the soluble oxygen of the H13 tool steel could be ignored at the w(CaO)/w(SiO2)=2 in the slag. The oxygen in the form of oxide inclusions depends on the total dissolution time (τ) of the inclusion into the slag. τ can be calculated by Eq. (30) [27].
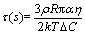 (30)
(30)
where ρ is the density of inclusion; R is the radius of inclusion; △C is the concentration difference between the inclusion and the slag; k is the Boltzmann constant; T is the temperature; α is the diameter of ionic; η is the viscosity of slag.
According to the calculation results of thermodynamics and kinetics, it can be assumed the composition of inclusion includes SiO2. If the radius of inclusion and the smelting temperature are constant, the relationship between τ and η/△C is shown as follows:
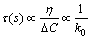 (31)
(31)
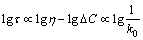 (32)
(32)
where k0 represents the removal rate of inclusions. Equations (31) and (32) qualitatively illustrates that the total dissolution time of the inclusion into the slag is inversely proportional to the removal rate of inclusion and the value of lg△C-lgη. The viscosity of the slag is calculated by Factsage 7.0 software, and the calculation results are shown in Table 6. In addition, the floating time of inclusion is related to the difference in concentration between the slag composition and the inclusions composition. It is assumed that the SiO2 content in inclusions is 50%, and the values of lg△C-lgη for experimental slags are calculated, as shown in Table 6.
The values of lg△C-lgη for slags 1 to 7 are between 3.19 and 3.60. Compared with the results reported by a previous study, which showed that the range of lg△C-lgη is 1.65 to 2.05 [14], it can be inferred that the removal rate of inclusion by the slags in the current study is prior to that of reported by PARK et al [14]. Consequently, most of oxide inclusions are floatable and removal, resulting in the small difference between the determined total oxygen content and the soluble oxygen content in H13 tool steel.
Table 6 Values of lg△C-lgη in different slags

4 Conclusions
In this study, the thermodynamic and kinetic calculations, as well as laboratory experiments, were performed to investigate the effect of slag composition on the limitation of deoxidation and the deoxidation rate in H13 tool steel with CaF2-MgO-CaO-Al2O3-SiO2 slags at 1873 K. The results are summarized as follows.
1) In the slag-steel reaction of H13 tool steel, the limitation of deoxidation depends on [Si]-[O] reaction rather than [Al]-[O] reaction, and besides basicity, other components in the slag have almost no effect on the limitation of deoxidation. The limitation of deoxidation decreases with the increase of basicity, and the limitation of deoxidation is the lowest at the basicity of 2.0.
2) The mass transfer models developed in the present study have been proven to be suitable for analysis of oxygen content in H13 tool steel smelted with CaF2-MgO-CaO-Al2O3-SiO2 slags with different w(CaO)/w(SiO2), w(Al2O3) and w(CaF2). The mass transport of O in the metal phase is the rate-controlling step for the kinetic model of deoxidation. The time for the deoxidation reaction to reach equilibrium is about 10 min, and the composition of slag has no effect on deoxidation rate.
3) In the case of w(CaO)/w(SiO2)=2.0 and w(CaF2)>50%, the total oxygen in H13 tool steel is less than 10×10-6. When the components of slag are w(CaF2)=51.5, w(MgO)=20.3%, w(Al2O3)=16.2%, w(CaO)=8.2%, w(SiO2)=3.8%, the total oxygen content in the steel reaches the lowest value (6×10-6). The slag composition provides a theoretical basis for the optimization of the slag of electroslag crucible furnace and ladle furnace.
Contributors
LI Shao-ying provided the concept and wrote the draft of manuscript. LI Bin participated in theoretical calculation. DUAN Sheng-chao provided the idea of experimental device. ZHAO Xing-ming provided the metal raw materials. GUO Jing conducted the literature review. GUO Han-jie edited the draft of manuscript.
Conflict of interest
LI Shao-ying, LI Bin, DUAN Sheng-chao, ZHAO Xing-ming, GUO Jing and GUO Han-jie declare that they have no conflict of interest.
References
[1] ZHANG Li-feng. State-of-the-art in the control of inclusions in tire cord steels-A review [J]. Steel Research International, 2006, 77(3): 158-169.
[2] PARK J H, KANG Y. Inclusions in stainless steels-A review [J]. Steel Research International, 2017, 88(12): 1700130-1700146.
[3] YAO Jun, QU Xuan-hui, RAFI-ud-din, HE Xin-bo, ZHANG Lin. Effect of inclusion on high cycle fatigue response of a powder metallurgy tool steel [J]. Journal of Central South University, 2012, 19(7): 1773-1779.
[4] SHI Cheng-bin, ZHENG Ding-li, GUO Bao-shan, LI Jing, JIANG Fang. Evolution of oxide-sulfide complex inclusions and its correlation with steel cleanliness during electroslag rapid remelting (ESRR) of tool steel [J]. Metallurgical and Materials Transactions B, 2019, 49(6): 3390-3402.
[5] ZHANG Li-feng, THOMAS B G, WANG Xin-hua, CAI Kai-ke. Evaluation and control of steel cleanliness review [C]// 85th Steelmaking Conference Proceedings. Warrendale: ISS-AIME, 2002: 431-452.
[6] KATO Y. Sanyo technical report [R]. Himeji: Sanyo Special Steel Co., Ltd., 1995. (in Japanese)
[7] HU Cheng-fei. Effect of oxygen content on fatigue properties of carburized gear steel [D]. Wuhan: Wuhan University of Science and Technology, 2019. (in Chinese)
[8] XU Jin, JIANG Xian-she, CHEN Zai-zhi, CHEN Jing-rong. Tool steel [M]. Beijing: Metallurgical Industry Press, 1998. (in Chinese)
[9] FU Jie, ZHU Jue. Changes of oxide inclusions during electroslag remelting [J]. Acta Metallurgy Sinica, 1964(7): 250-261. (in Chinese)
[10] SHI Cheng-bin, CHEN Xi-chun, GUO Han-jie, ZHU Zi-jiang, REN Hao. Assessment of oxygen control and its effect on inclusion characteristics during electroslag remelting of die steel [J]. Steel Research International, 2012, 83(5): 472-486.
[11] WEI Ji-he, LIU Zong-yuan. Study on oxygen transfer of slag based on CaF2+Al2O3 and CaF2+Al2O3+CaO for electroslag remelting [J]. Acta Metallurgy Sinca, 1994, 30(8): 350-359. (in Chinese)
[12] CHEN Xi-chun, SHI Cheng-bin, GUO Han-jie, WANG Fei, REN Hao, FENG Di. Investigation of oxide inclusions and primary carbonitrides in Inconel 718 superalloy refined through electroslag remelting process [J]. Metallurgical and Materials Transactions B, 2012, 43(6): 1596-1607.
[13] DUAN Sheng-chao, SHI Xiao, ZHANG Man-cang, LI Bin, YANG Wen-sheng, WANG Fei, GUO Han-jie, GUO Jing. Effect of slag composition on the deoxidation and desulfurization of Inconel 718 superalloy by ESR type slag without deoxidizer addition [J]. Metallurgical and Materials Transactions B, 2020, 51(2): 353-364. DOI: https://doi. org/10.1007/s11663-019-01729-3.
[14] PARK S, PARK J H. Effect of physicochemical properties of slag and flux on the removal rate of oxide inclusion from molten steel [J]. Metallurgical and Materials Transactions B, 2016, 47B (12): 3225-3230.
[15] CHANG Z, SHI X F, CONG J Q. Study on mechanism of oxygen increase and countermeasure to control oxygen content during electroslag remelting process [J]. Ironmaking & Steelmaking, 2014, 41(3): 182-186.
[16] DUAN Sheng-chao, SHI Xiao, MAO Ming-tao, YANG Wen-sheng, HAN Shao-wei, GUO Han-jie, GUO Jing. Investigation of the oxidation behaviour of Ti and Al in Inconel 718 superalloy during electroslag remelting [J]. Scientific Reports, 2018, 8(1): 1-13.
[17] HUANG Xi-hu. Principles of metallurgy [M]. Beijing: Metallurgical Industry Press, 2011. (in Chinese)
[18] DUAN Sheng-chao, GUO Xiao-long, GUO Han-jie, GUO Jing. A manganese distribution prediction model for CaO-SiO2-FeO-MgO-MnO-Al2O3 slags based on IMCT [J]. Ironmaking & Steelmaking, 2017, 44 (3): 168-184.
[19] LEI Jia-liu, ZHU Hang-yu, ZHAO Dong-nan, XUE Zheng-liang. Generation mechanism of MgO and Al2O3 inclusions in 51CrV4 spring steel based on the ion–molecule coexistence theory [J]. Metals, 2019, 9(8): 830-836.
[20] CHEN Jing-feng, DU Xiao-jian, CHANG Guo-dong, MA Jun-peng, XING Ji-bing. Erosion mechanism and improvement measures for magnesia-calcium brick lining of AOD furnace [C]// Proceedings of the 9th China Steel Annual Conference. Beijing, 2013: 1-4. (in Chinese)
[21] HOU Dong, WANG De-yong, QU Tian-peng, TIAN Jun, WANG Hui-hua. Kinetic study on alloying element transfer during an electroslag remelting process [J]. Metallurgical and Materials Transactions B, 2019, 50 (6): 3088-3102.
[22] PARK J, SRIDHAR S, FRUEHAN R J. Kinetics of reduction of SiO2 in SiO2-Al2O3-CaO slags by Al in Fe-Al(-Si) melts [J]. Metallurgical and Materials Transactions B, 2014, 45B(8): 1380-1388.
[23] SHI Cheng-bin, CHO J W, ZHENG Ding-li, LI Jing. Fluoride evaporation and crystallization behavior of CaF2-CaO-Al2O3-(TiO2) slag for electroslag remelting of Ti-containing steels [J]. International Journal of Minerals, Metallurgy and Materials, 2016, 23(6): 627-636.
[24] CHEN Jia-xiang. Handbook of common data sheets for steelmaking [M]. 2nd eds. Beijing: Metallurgical Industry Press, 2010. (in Chinese)
[25] LI Shi-jian, CHENG Guo-guang, MIAO Zhi-qi, CHEN Lie, LI Cheng-wei, JIANG Xin-yan. Kinetic analysis of aluminum and oxygen variation of G20CrNi2Mo bearing steel during industrial electroslag remelting process [J]. ISIJ International, 2017, 57(12): 2148-2156.
[26] NI Peng-yuan, TANAKA T, SUZUKI M, NAKAMOTO M. A kinetic model of mass transfer and chemical reactions at a steel/slag interface under effect of interfacial tensions [J]. ISIJ International, 2019, 59(5): 737-748.
[27] VALDEZ M, SHANNON G, SRIDHAR S. The ability of slags to absorb solid oxide inclusions [J]. ISIJ International, 2006, 46(3): 450-457.
(Edited by HE Yun-bin)
中文导读
1873 K下CaF2-MgO-CaO-Al2O3-SiO2渣系对H13模具钢的脱氧研究
摘要:本文根据热力学和动力学理论,结合实验室实验,研究了1873 K下CaF2-MgO-Al2O3-CaO-SiO2渣系成分对H13工具钢脱氧极限的影响。理论计算结果与实验结果基本一致。研究结果表明,[Si]-[O]反应为脱氧控制反应,随着炉渣碱度的增大,脱氧极限降低。当碱度为2.0时,脱氧极限最低。在碱度为2.0且CaF2含量大于50%的条件下,脱氧极限小于10×10-6,且该值不受炉渣中Al2O3和CaF2的含量的影响。氧在金属相中的传质过程是脱氧反应的限制性环节,因此,脱氧反应速率不受渣系成分的影响。基于上述结果,设计了优化的炉渣成分,即 51.5% CaF2,20.3% MgO,16.2% Al2O3,8.2% CaO和3.8% SiO2。在此成分条件下,H13钢中的全氧含量可以从25×10-6降低到6×10-6。
关键词:脱氧; H13工具钢; 渣-钢界面反应; 炉渣优化
Foundation item: Project(18SYXHZ0069) supported by the Science and Technology Program of Sichuan Province, China; Projects(51974139, 51664021) supported by the National Natural Science Foundation of China
Received date: 2020-06-28; Accepted date: 2020-11-17
Corresponding author: GUO Han-jie, PhD, Professor; Tel: +86-10-62334964; E-mail: guohanjie@ustb.edu.cn; ORCID: https://orcid.org/ 0000-0002-6493-8853

