
Preparation and properties of PAn/ATTP/PE conductive composites
QIU Jian-hui(邱建辉)1, FENG Hui-xia(冯辉霞)1,2,3
1. Department of Machine Intelligence and Systems Engineering, Faculty of Systems Engineering,
Akita Prefectural University, Akita 015-0055, Japan
2. College of Petrochemical Technology, Lanzhou University of Technology, Lanzhou 730050, China
3. State Key Laboratory of Gansu New Nonferrous Metal Materials, Lanzhou 730050, China
Received 10 April 2006; accepted 25 April 2006
Abstract: Polyaniline/Attapugite/ PE(PAn-ATTP/PE)composites containing particles with core-shell structure were obtained via the two-step blending processs. The experimental condition is as follows: Organo-attapulgite and PAn was obtained by modifying attapulgite with laury benzenesulfonic acid sodium salt and, then added to PE. The electrical conductivity,structure and properties of the composites were studied. Under the function of shear stress, core-shell structure particles with ATTP as the core and PAn as the shell were formed in the composites. The structure of PAn-ATTP/PE composites were characterized by FTIR,XRD,SEM, etc, respectively. The effects of concentration of doping agent on the conductivity and mechanical property of the composites were investigated. The mechanical properties and impact fracture surface of the ternary composites were studied by means of the tensile tester, SEM, etc. The results show that polyaniline encapsulated ATTP enhances the strength of the PE. And the conductivity of PAn-ATTP/PE composites of is improved effectively when polyaniline encapsulated ATTP is added. The composite have good conductivity when 10% polyaniline encapsulated ATTP is added.
Key words: core-shell structure; Polyaniline; Attapugite; composites
1 Introduction
In the last two decades, organic–inorganic nanometer composites have attracted great interest, both in academia and industry, because of their remarkable improvement in mechanical and other properties when compared with plain polymer or conventional micro and macrocomposites, such as high modulus, increased strength and heat resistance[1,2].Polymers are filled with an inorganic component to increase their strength or impact resistance,to reduce their electrical conductivity and permeability of oxygen and other gases. For example, polymer/montmorillonite (MMT) nanocomposites, such as PA6/MMT, PET/ MMT, PEO/MMT, PMMA/MMT, have been prepared successfully by many researchers.
These improvements increase as a function of the length/diameter ratio (aspect ratio) and dispersion of the inorganic phase and are limited in conventional filled polymers by the distinct macroscopic separation of the two phases. This is because they can intercalate MMT layers and finally form intercalated or exfoliated nanocomposites.
This restriction is overcome in a new class of materials known as polymer-layered silicate nanocomposites (PLSN) because the particles in their dispersed phase are of the order of a few nanometers. Interactions between macro molecules and layered silicates have been studied for many years. However, for polyolefin polymers, such as PP and PE, which do not include any polar group in their backbone, silicate layers even modified by nonpolar long alkyl groups are polar and incompatible with polyolefin.
In our studies, another clay that is different from MMT, attapulgite[3,4], is adopted and filled in the PAn and PE matrix. Attapulgite (or palygorskite as it often called) is a crystalline hydrated magnesium aluminum silicate with unique three-dimensional structure and has a fibrous morphology. Attapulgite has the structural formula Si8O20Mg5(Al)(OH)2(H2O)4·4H2O, and its ideal structure is studied by BRADLEY early in 1940. The distinguishing feature of its structure is that the Si—O tetrahedra form long strips, each an amphibole unit wide,on alternate sides of the oxygen sheet in a manner which confers a regular corrugated Si—O structure. The formulas are written as such to indicate the two types ofwater present, magnesium coordinated water and adsorbed water. The structure of the mineral results in zeolite-like channels, which are approximately 0.37 nm×0.60 nm and 0.56 mm×1.10 nm wide, respectively. Because of its structural morphology, attapulgite has received considerable attention with regard to the adsorption of organics on the clay surface, but there are only a few reports about its use in the nanocomposites.
In this study, we report a new kind of PE/PAn-clay nanocomposites and a new method to prepare the nanocomposites by the two-step blending processs. The strong interaction caused by the suspend reaction can improve the dispersion effect of the attapulgite in the PAn and PE matrix. The corresponding properties of the resulting nanocomposites were investigated.
2 Experimental
2.1 Materials
PE used in this study was purchased from Japan;Attapulgite(ATTP) clay was obtained from Toshin Chemicals Limited Company (Japan);laury benzenesulfonic acid sodium salt,aniline, (NH4)2S2O8 and hydrochloric acid were all analytical grade.
2.2 Experiment methold
2.2.1 Preparation of producing organic Attapulgite
The mixtures of Attapulgite, aniline and laury benzenesulfonic acid sodium salt (LAS)were added in a water solution of hydrochloric acid at room temperature with vigorous stirring for 24 h. The amount of added LAS was 1.0 mmol to 1 g of MMT. The LAS and aniline modified ATTP in a water solution of hydrochloric acid It is named as a water solution of O-ATTP.
2.2.2 Preparation of PAn /O-ATTP nanocomposites
After 24 h with vigorous stirring, a radical initiator, ammonium persulfate,(NH4)2S2O8, was added to the mixture. Finally the mixture was kept stirring for 24 h. The products were filtered and washed with a water solution of hydrochloric acid and acetone alternant for three times. Obtained hybrid PAn-O-ATTP was dried at 50 ℃ under vacuum[5-8].
2.2.3 Preparation of PAn-ATTP/PE nanocomposites
There are two methods .The experimental condition of the first method is as follows: The dried PAn-O-ATTP was mixed with PE in mixer to obtain PAn-ATTP/PE. The PAn-O-ATTP contents were varied from 5% to 30% by weight. The experimental condition of the second method is as follows: The PE were added in a water solution of hydrochloric acid which it contains O-ATTP at room temperature with vigorous stirring for 24 h. Then, a radical initiator, ammonium persulfate (NH4)2S2O8, was added to the mixture. Finally the mixture was kept stirring for 24 h. The products were filtered and washed with a water solution of hydrochloric acid and acetone alternant for three times. Obtained composites PE-PAn-O-ATTP was dried at 50 ℃ under vacuum. The PAn-O-ATTP contents were About 10% by mass.
The two kinds of obtained composites were hot-press molded in a parallel plate press at a temperature of 160 ℃ with a residence time of 2.5 min at 20 MPa. Specimens about 1 mm in thickness were cut from the plaques for the different measurements realized in the present study, as shown in Fig.1.
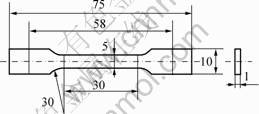
Fig.1 Shape and measurement of specimens(mm)
2.3 Characterization
Wide-angle X-ray spectra were recorded with a PANalytical X’Pert ED; diffracto-meter; the X-ray beam was nickel-filtered Cu Kα(λ=0.154 2 nm) radiation oper-
ated at 40 kV and 100 mA; corresponding data were collected from 2? to 10? at a scanning rate of 1 (?)/min; FOURIER transform infrared spectra were recorded using a JASCO Corporation MFT-2000 FT-IR spectrometer with the Powder sample; The morphology of the ATTP, O-ATP, PAn-O-ATTP and PAn-ATTP/PE were inspected in a scanning electron microscope (SEM) named Hitachi Ltd S-4300. Powder samples were dispersed in the adhesive tape of conductivity before the SEM examination; Tensile test was carried out using a universal testing machine Instron-3360 tester with a speed of 10 mm/min at room temperature. E-modulus, tensile strength and elongation at break were evaluated from stress–strain data.
3 Results and discussion
3.1 Effect of surface modification on ATTP
Fig.2 shows the XRD patterns of ATTP, O-ATTP and PAn-O-ATTP. The peaks at 2θ=8.3? correspond to the primary diffraction of the (110) planes of the clay respectively. The results clearly show that the one-plane characteristic peaks for pure ATTP and treated ATTP do not change at all. That is to say, surface treatment of ATTP has no evidently effects on its crystal structure. But from the results of FT-IR and DSC, the covalent
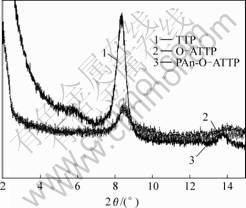
Fig.2 XRD patterns of ATTP, O-ATTP and PAn-O-TAAP
bond has formed in O-ATTP and PAn-O-ATTP.
SEM micrographs of ATTP, O-ATTP and PAn-O-ATTP are shown in Fig.3. From Fig.3(a), we can see that the ATTP has a fibrous morphology and its diameter is about 20 nm and length is 300-1 000 nm. By the way of surface modification using the mixtures (together with aniline and LAS were added in a water solution of hydrochloric acid), as shown in Fig.3(b), an average diameter of O-ATTP is approximately 40 nm, and the small aggregates are still existent though the 24 h dispersion was adopted after surface modification. From Fig.3(c), we can see the ATTP clay re-aggregated when they were covered with the PAn, the polymerization of PAn may cause core-shell structure to shape.
SEM micrographs of PE, PE-PAn-O-ATTP and PAn-O-ATTP/PE are shown in Fig.4. From Fig.4(a), we can see that the PE has a granule morphology. By way of
surface modification using the mixtures (together with O-ATTP were added in a water solution of hydrochloric acid which contains a radical initiator), as shown in Fig.4(b), an average diameter of PAn-O-ATTP is about 100 nm. It distributes well on the surface of the PE. From Fig.4(c), we can see the PAn-O-ATTP re-aggregated when they were immingled with the PE.
3.2 PAn chain conformation in composites[9-11]
From the XRD and SEM results we can presume that in the PAn-composite the PAn is an extended chain conformation and we obtain the direct proof from FTIR spectra.FT-IR spectra of the synthesized Pan-O-ATTP nanocomposites are shown in Fig.5, together with those of pristine ATTP and pure PAn. The FT-IR spectra also confirm the presence of the conductive form of PAn in the ATTP. In the FT-IR spectra, the band at 1 570 cm-1 is assigned to the C==N stretching mode. The peaks at
1 300 and 1 250 cm-1 are associated with the C—N stretching modes. These peaks are ascribed to the formation of PAn. The strong peak at 988 cm-1 are the characteristic vibrations of ATTP.
The FTIR spectra of ATTP, ATTP-PE, PAn-O-ATTP/PE, PE-PAn-ATTP and ATTP-PAn-PE composites are shown in Fig.6. The appearance of absorption bands at 1 570 cm-1 , 1 300 cm-1 and 1 250 cm-1 attributed to C==N and C—N of the PAn bends gives direct evidence for the reaction between ATTP and PAn comparing with the spectra of ATTP (Fig.5). The disappearance of Si—OH deformation at 988 cm-1 compared with the spectra of ATTP (Fig.6) also proves
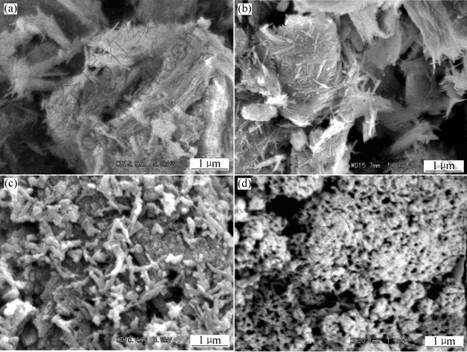
Fig. 3 SEM micrographs of ATTP, O-ATTP, PAn-O-ATTP and Pan: (a) ATTP; (b) O-ATTP; (c) PAn-O-ATTP; (d) Pan
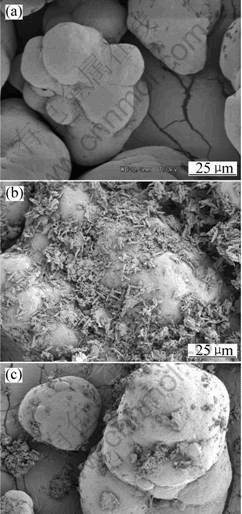
Fig. 4 SEM micrographs of PE(a), PE-PAn-O-ATTP(b) and PAn-O-ATTP/PE(c)

Fig. 5 IR spectra of pristine ATTP, pure PAn and Pan-O-ATTP
the participation of Si—OH in the formation.Theintensities of these peaks corresponding to PAn in the spectrum of PE-PAn-ATTP became weaker than those of PAn-O-ATTP. This would be expected because a less amount of PAn has been synthesized on the surface of
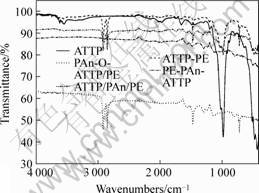
Fig. 6 IR spectra of pristine ATTP and composites
PE than for PAn-O-ATTP.
3.3 Conductivity and mechanical properties of nanocomposites
The room temperature conductivity of PE-PAn- ATTP composites has a relatively high conductivity in comparison with other mixtures of PAn-ATTP and PE.
The stress-strain curves of the composites are shown in Fig.7. Yield phenomenon occurs in the matrix of PE when the strain is 15%, and it does not rupture up to 200% strain of PE. The results clearly show that PE is a typical ductility materials. However, when 10% (PAn+ATTP) was added to the PE, the new composites lose their ductilities and their rupture-stresses decline sharply. Especially, the tensile intensities and rupture-stresses of the composites becomes very low.
Fig.8 shows SEM micrographs of the rupture- surface of the new composites. From Fig.8(a), we can see that PAn and ATTP agglomerate on the matrix of PE when it is prepared by physical mixture. This causes the mechanical properties of the composites to be lower. In
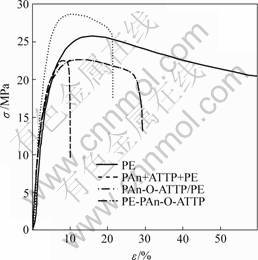
Fig.7 Stress-strain curves of composites
this study, we obtain PE-PAn-ATTP composites containing particles with core-shell structure via the two-step blending process. Under the function of shear stress, core-shell structure particles with ATTP as the core and PAn as the shell were formed in the composites. Organo-attapulgite and PAn was deposited to PE. The tensile intensity is 28.69 MPa and 13% improvement is obtained in the new kinds of composites (PE-PAn-ATTP) compared with pure PE. Therefore, this method of preparation of the new composites can improve conductivity. Moreover, it can increase the intensity of the new composites. As shown in Fig.8(b), PAn-ATTP is dispersed very well in the PE matrix, and agglomerate phenomena does not occur.
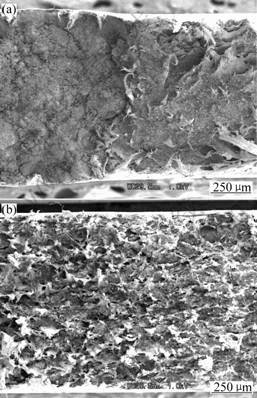
Fig.8 SEM micrographs of rupture-surface of composites:(a) ATTP/PAn/PE; (b) PE -PAn-ATTP
4 Conclusions
The new composites consisting of PAn-O-ATTP composite (containing particles with core-shell structure) and PE by the new method(the two-step blending processs) are synthesized. The XRD analysis proves that surface modification has no effect on the morphology of ATTP. The SEM results reveal an enhancement in the dispersing for the PE-PAn-O-ATTP composite relative to pure PE. The tensile intensity value of PE-PAn-O-ATTP (10% PAn-O-ATTP) composites is 28.69 MPa and 13% improvement is obtained in the new kinds of composites (PE-PAn-ATTP) compared with pure PE. Consequently, this study provides a new and effective method for the preparation of functionality composites.
References
[1] GIANNELIS E P, KRISHNAMOORTI R, MANIAS E. Polymer-silica nanocomposites: modeled systems for confined polymer and polymer brushs[J]. Advances in Polymer Science,1999,138:107-147.
[2] ALEXANDRE M, DUBOIS P. Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials[J]. Materials Science and Engineering, 2000, 28: 1-63.
[3] SHEN L, LIN Y J, DU Q G, ZHONG W, YANG Y L. Preparation and rheology of polyamide-6/attapulgite nanocomposites and studies on their percolated structure[J]. Polymer, 2005, 46(15, 11): 5758-5766.
[4] FAIREY R C, JENNINGS B R.Electrooptical study of the effect of surfactant on attapulgite clay sol stability[J].Journal of Colloid and Interface Science, 1982, 85(1): 205-215.
[5] COTTEYIEILLE D,LEMEHOUTE A, CHALLIOUI C, MIREBEAU P,DEMAG J N.Industrialapplications of polyaniline[J].Synthetic Metals,1999, 101: 70-704.
[6] PHAWAN S K,SINGH N,VEN R S.Shielding effective- nessofconductingpolyanilinecoatedfabricsaf101GHz[J]. Synthe- tic Metals, 2002, 125: 389-393.
[7] FAEZ R,MARTIN I M,PAOLIMA De,REZENDE M C. Influence of processing time and composition the microwave absorption of EPD M/Pan blends[J]. Applied Polym Sci, 2002, 83: 1568-1575.
[8] FAN J H, WAN M X, ZHU D B. Synthesis and characterization of water-soluble conducting copolymer poly (aniline-co-o- aminobenzenesulfonic acid)[J]. Journal of polymer Science(Part A): Polymer Chemistry, 1998 (36): 3031-3019.
[9] KUANG Y Z. Review of Conductive Polyaniline[J]. Fine Chemical Intermdiates, 2004, 34 (4): 18-20.
[10] ZHANG Q H, JIN H F, WANG X H, et al. Morphology of conductive blend fibers of polyaniline and polyamide-11[J]. Synthetic Metals, 2001, 123: 481-485.
[11] XIA H S, WANG Q. Synthesis and characterization of conductive polyaniline nanoparticles through ultrasonic assisted inverse microemulsion polymerization[J]. Journal of Nanoparticle Research, 2001, 3: 401-411.
(Edited by CHEN Ai-hua)
Corresponding author: QIU Jian-hui; Tel.: +81-184-27-2134; fax: +81-184-27-2188; E-mail: qiu@akita-pu.ac.jp