
Combined effect of amino and carboxyl group in α-alanine on
seeded precipitation of sodium aluminate solution
L? Bao-lin(吕保林), CHEN Qi-yuan(陈启元), YIN Zhou-lan(尹周澜), HU Hui-ping(胡慧萍)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 6 October 2008; accepted 12 December 2008
Abstract: α-alanine was adopted as a new additive to elucidate the seeded precipitation mechanism of sodium aluminate solution. α-alanine has the inhibitory effect at the initial period of reaction, but the favorable effect in subsequent reaction. The combined effect of amino and carboxyl group in α-alanine was confirmed by investigating the effect of propionic acid, ethamine and the mixture of propionic acid and ethamine (mole ratio 1?1) on the precipitation of sodium aluminate solution, respectively. The inhibitory effect derives from the adsorption of amino or carboxyl group in α-alanine on the active surface sites of gibbsite, which was confirmed by the alleviating inhibitory effects of propionic acid, ethamine and α-alanine due to the double crystal seed mass. The semi-quantitative IR spectrum analysis of the relative concentrations of Al2O(OH)62- with the band at about 550 cm-1 and polynuclear aluminate ion with the bands at about 880 cm-1 and 635 cm-1, indicates that the dynamic balance among some aluminate species present in sodium aluminate solution is broken due to the addition of α-alanine, thus resulting in the change of the seeded precipitation ratio of sodium aluminate solution.
Key words: α-alanine; sodium aluminate solution; seeded precipitation
1 Introduction
It is well known that the low seeded precipitation ratio of sodium aluminate liquors in Bayer process has bothered researchers and producers of alumina for many years. Considerable efforts and methods have been devoted to elucidating the precipitation mechanism of sodium aluminate solution and intensifying the seeded precipitation process of sodium aluminate solution. Optimizing operating conditions, activating seeds and using additives are the main measures which have been applied in the production of gibbsite. Among them, using additives as an intensifying method without changing the present equipments and technological processes is considered to be one of the best methods. So far, quite a few works have been mostly focused on organic polymers[1-4] and surfactants[5-9] to enhance the seeded precipitation ratio of sodium aluminate solution. However, these work only encompassed some technological processes, and the seeded precipitation mechanisms were not uncovered yet because of the complex molecule structure of these compounds in solution.
In this work, α-alanine was adopted as a new additive with special complex molecule structure for elucidating the seeded precipitation mechanism of sodium aluminate solution. The combined effect of amino and carboxyl group in α-alanine on the seeded precipitation of sodium aluminate solution was studied. A possible new method is offered for highlighting investigations to intensify precipitation of gibbsite from sodium aluminate solution containing additives with multiple functional groups.
2 Experimental
2.1 Materials and apparatus
Distilled water (single distilled water) was self made. Aluminate hydroxide (industrial grade, supplied by Zhengzhou Research Institute of Aluminum Corporation of China Limited) was washed, and then dried. Sodium hydroxide (analytical reagent, Shantou Xilong Chemical Company of China) was adopted in the experiments. Crystal seeds (obtained from Zhengzhou Research Institute of Aluminum Corporation of China Limited) were washed, dried and then passed through sieve of 45 mm, and the <45 mm seeds were mixed homogeneously for experiments. α-alanine (chemically pure, from Chemical Reagent Corporation of China Limited), propionic acid (chemically pure, from Zhangjing Chemical Reagent Company, China) and ethamine (Chemically pure, from Shanghai Chemical Reagent Company, China) were adopted for the experiments. Precipitation tank, with a volume of 1 L and valid volume of 0.8 L, is a stainless steel vessel. The tank is set in an isothermal bath.
2.2 Methods
A certain volume and concentration of sodium aluminate liquors were added into precipitation tank which was preheated to a constant temperature. The additive of precise dosage was added as the liquor reached a constant temperature. Crystal seeds were added and the reaction time was recorded after 20 min isotherm process. Samples were taken periodically from the precipitation tank and centrifuged. Clear liquors were used to analyze the content of alumina and alkali. 0.25 mL of 400 g/L analytically pure sodium nitrate solution as an internal standard substance was added into 5 mL clear liquor[10], then mixed uniformly.
The metallurgical industry standard of YB-817-75 was adopted for the analysis of chemical components. This is a titrimetric method based on the work of WATTS and UTLEY[11].
Nicolet 6700 Fourier Transform Infrared spectrometer was adopted for semi-quantitative spectrum analysis of various forms of aluminate ions in sodium aluminate solution, which were obtained at 2 cm-1 resolution and recorded at ambient temperature of (25±1 ℃). At the same time, the blank experiment was performed.
2.3 Conditions of seeded precipitation
Experiments were performed under the conditions of alkali (Na2Ok) concentration 140 g/L, initial molecular ratio (αk, Na2Ok to Al2O3) 1.45, temperature (75±0.2) ℃, agitation rate 100 r/min, seed mass ratio(Ks) 0.25 or 0.5, and precipitation time 10 h.
3 Results and discussion
3.1 Effect of α-alanine on seeded precipitation rate of sodium aluminate solution
The effect of α-alanine on the seeded precipitation rate of sodium aluminate solution is shown in Fig.1. It is indicated that, compared with the blank, α-alanine at certain concentrations has the inhibitory effect at the initial period of reaction, but accelerates the precipitation process in the subsequent reaction time. Moreover, the inhibitory effect becomes stronger and the inhibitory time is reduced with the increase of concentration of α-alanine.

Fig.1 Effect of α-alanine on seeded precipitation rate of sodium aluminate solution
3.2 Mechanism of accelerating precipitation
3.2.1 Relationship between propionic acid, ethamine and α-alanine
The schematic diagram of the relationship between propionic acid, ethamine and α-alanine is shown in Fig.2. Propionic acid and ethamine can be considered as two substances transformed from α-alanine when the bond A or B is cut. Therefore, the effects of propionic acid and ethamine and their mixture (mole ratio 1?1) are investigated respectively for confirming the effectively active group in α-alanine or the combined effect of amino and carboxyl group in α-alanine.

Fig.2 Schematic diagram of relationship between propionic acid, ethamine and α-alanine
3.2.2 Effect of additive on seeded precipitation rate of sodium aluminate solution
The effects of propionic acid, ethamine and their mixture on the seeded precipitation rates of sodium aluminate solutions are shown in Fig.3. It is clear that propionic acid, ethamine and the mixture at certain concentrations all show inhibitory effects on sodium aluminate solution to some extent. According to this, combined with Fig.1, it can be concluded that the increase of the seeded precipitation rate of sodium aluminate solution in subsequent reaction time results from the combined effect of amino and carboxyl group inα-alanine. Because Al (OH)4- is a main aluminate ion in sodium aluminate solution under the experimental conditions[12], the possible interaction between α-alanine and Al (OH)4- could occur due to the formation of hydrogen bond, which leads to the high local concentration of Al(OH)4- in solution, thus promoting the precipitation. The schematic diagram of the possible interaction mode between α-alanine and Al(OH)4- is shown in Fig.4. However, further investigations are needed before obtaining accurate conclusion.

Fig.3 Effect of additive on seeded precipitation rate of sodium aluminate solution: (a) Propionic acid; (b) Ethamine; (c) Mix- ture of propionic and ethamine

Fig.4 Schematic diagram of possible interaction between α-alanine and Al(OH)4-
3.3 Mechanism of inhibitory precipitation
The effects of additives of 4.2×10-3 mol/L on the seeded precipitation ratio of sodium aluminate solution at various seed mass ratios are shown in Fig.5. Figs.5(a1)- (a4) show the crystallization curves with time when the seed mass ratio is 0.25; for comparison, the crystallization curves with time are shown in Figs.5(b1)- (b4) with seed mass ratio of 0.5. As can be seen from Fig.5, the inhibitory effects of α-alanine, propionic acid, ethamine and the mixture on the precipitation process of sodium aluminate solutions all become weak when the seed mass ratio changes from 0.25 to 0.5. In a similar study, ZENG et al[12] investigated the inhibitory effect of mannitol on the gibbsite crystallization from sodium aluminate solution at various seed mass ratios, and concluded that the weaker inhibitory effect of mannitol at higher seed mass ratio resulted from the insufficiency of the same mass mannitol to cover all the active sites on the surface of gibbsite.

Fig.5 Effect of additive of 4.2×10-3 mol/L on seeded precipitation rate of sodium aluminate solution at various seed mass ratios: (a1)-(a4) 0.25; (b1)-(b4) 0.5
Therefore, it can be concluded that the inhibitory effect of α-alanine results from the adsorption of single carboxyl or amino group in α-alanine on the surface of gibbsite at the initial period of reaction.
3.4 Semi-quantitative analysis of characteristic peak of aluminate ion
The IR spectra of sodium aluminate solution with and without sodium nitrate as an internal standard substance are shown in Fig.6. As can be seen from Fig.6, the absorption band at about 720 cm-1 is attributed to the asymmetric Al—O stretching vibration of Al(OH)4-[13], the band at about 550 cm-1 is the Al—O—Al vibration of the dimer Al2O(OH)62-[14], the band at about 635 cm-1 and about 880 cm-1[15] may be associated with a polynuclear aluminate ion formation, and the band at about 1 380 cm-1 is the characteristic peak of sodium nitrate[10]. Fig.6 shows that, for the characteristic peaks of different aluminate ions present in sodium aluminate solution, no apparent chemical shifts are observed in 400-4 000 cm-1 region compared with the blank after sodium nitrate in proper dosage as an internal standard substance was added into sodium aluminate solution. Therefore, it is feasible to choose sodium nitrate at proper concentration as an internal standard substance.

Fig.6 FT-IR spectra of sodium aluminate liquor with (a) or without (b) addition of sodium nitrate
As discussed in section 3.1 (shown in Fig.1), the addition of α-alanine at proper concentration reduces the precipitation rate of sodium aluminate solution at the initial period of reaction but accelerates the precipitation process in subsequent reaction time. What change happens about the relative concentration of the dimer or the polynuclear aluminate ion which could be beneficial to the formation of growth unit[16] in the sodium aluminate solution with α-alanine?
In order to evaluate the change of the relative concentration of the dimer or the polynuclear aluminate ion in sodium aluminate solution, R that is the peak value ratio of characteristic peak of aluminate species to that of 1 380 cm-1 of sodium nitrate was semi- quantitatively calculated.
The changes of the peak value ratios of the absorption band at about 550 cm-1 of the dimer and the bands at about 880 cm-1 and 635 cm-1 of the polynuclear aluminate ion are shown in Fig.7, when 4.2×10-3mol/L α-alanine is added into sodium aluminate solution. It can be found from Fig.7 that the peak value ratios R increase
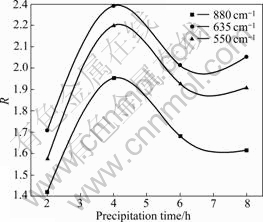
Fig.7 Change of R in sodium aluminate solution with 4.2× 10-3mol/L α-alanine (R: Peak value ratio of characteristic peaks of aluminate species to that of 1 380 cm-1of sodium nitrate)
as the precipitation time increases from 2 to 4 h, then decrease during the period of 4 to 8 h. In the whole, the peak value ratios R increase during the period of 2 to 8 h, which reveals that the concentrations of the dimer and the polynuclear aluminate ion increase in the given experimental time.
The changes of the peak value ratios of the band at about 550 cm-1 of the dimer and the bands of the polynuclear aluminate ion at about 880 cm-1 and 635 cm-1 are shown in Fig.8 for the blank. It can be found from Fig.8 that, the peak value ratios R decrease as the precipitation time increases from 2 to about 6 h and then increase at 8 h. But the total tendency of R decreases during the period of 2 to 8 h, which indicts that the concentrations of the dimer and the polynuclear complex decrease in the experiments.

Fig.8 Change of R in sodium aluminate solution without α-alanine
It is also observed that the concentrations of the dimer with the band at about 550 cm-1 and the polynuclear aluminate ion with the bands at about 880 cm-1 and 635 cm-1 (in Fig.8) are lower than those for the blank (in Fig.8) at the initial period of reaction, which corresponds to the change of the seeded precipitation ratio of sodium aluminate solution (shown in Fig.1) at the same time. But the inverse tendency occurs afterwards, which is also similar with the change of the seeded precipitation ratio of sodium aluminate solution (shown in Fig.1).
Therefore, it can be concluded that the addition of α-alanine at proper dosages into sodium aluminate solution leads to the break of dynamic balance among some aluminate species present in sodium aluminate solution, thereby resulting in the change of the seeded precipitation ratio of sodium aluminate solution.
In addition, it can be found from Fig.7 and Fig.8 that it is interesting that oscillation phenomena of R are observed during the whole experiment, which is consistent with our previous results[17].
In summary, there are two inverse interactions among α-alanine and aluminate ions and gibbsite particles simultaneously existed in sodium aluminate solution with α-alanine at certain concentrations. One is an inhibitory interaction which results from the adsorption of the carboxyl or amino group in α-alanine on the surface of gibbsite and is dominant at the initial period of reaction; the other is a favorable interaction with a predominance afterwards, which could deprive the formation of hydrogen bond between α-alanine and aluminate ions. The two inverse interactions lead to the break of dynamic balance of some aluminate ions, therefore resulting in the change of the seeded precipitation rate of sodium aluminate solution.
4 Conclusions
1) The effect of α-alanine on the precipitation rate of sodium aluminate solution is inhibitory at the initial period of reaction, but is favorable in subsequent reaction time.
2) The inhibitory effect of α-alanine results from the absorption of carboxyl or amino group in α-alanine on the surface of gibbsite.
3) There is a combined effect between carboxyl or amino group in α-alanine.
4) Two inverse interactions could simultaneously occur among α-alanine and aluminate ions and gibbsite particles in sodium aluminate solutions, which results in the change of the concentration of some aluminate ions.
References
[1] ROE W J, PERISHO J L. Use of polymers in alumina precipitation in the Bayer process of bauxite beneficiation: US4608237 [P]. 1986-08.
[2] WU Yu-sheng, YU Hai-yan, YANG Yi-hong, BI Shi-wen. Effects of additives on agglomeration and secondary nucleation in seed precipitation in sodium aluminate solution [J]. Journal of Chemical Industry and Engineering (China), 2005, 56(12): 2434-2439. (in Chinese)
[3] YIN Zhou-lan, JING Ye-ling, CHEN Qi-yuan, ZHANG Ai-min. Effect of polymers on seed precipitation of sodium aluminate solution [J]. The Chinese Journal of Nonferrous Metals, 2007, 17(6): 1002-1007. (in Chinese)
[4] MAHONEY R P, SCHNIEDERS JR W B. Trihydrate crystal modifier for the Bayer process: US 0159936 [P]. 2002-08.
[5] OWEN D O, DAVIS D C. Use of surfactants in alumina precipitation in the Bayer process: US 4737352 [P]. 1988-08.
[6] CHEN Feng, ZHANG Bao-yan, BI Shi-wen, XIE Yan-li. Effect of anionics-oily additive on seed precipitation from sodium aluminate solution [J]. Journal of Northeastern University (Natural Science), 2004, 25(6): 606-609. (in Chinese)
[7] ZHAO Su, BI Shi-wen, DING Xiang-qun, TONG Zhi-fang. Influence of novel surfactants on the physicochemical properties of sodium aluminate solution relevant to the aluminium hydroxide precipitation process [J]. Mineral Processing and Extractive Metallurgy, 2005, 114: 53-56.
[8] YANG Yi-hong, BI Shi-wen, XIE Yan-li. Effects of surfactant additives on the seed precipitation in sodium aluminate aguatic solution [J]. Journal of Northeastern University (Natural Science), 2002, 23(11): 1076-1078. (in Chinese)
[9] CHEN Feng, ZHANG Bao-yan, BI Shi-wen, CHEN Yu-guo. Effect of non-ionic oily additive on precipitate from sodium aluminate solution [J]. Journal of Northeastern University (Natural Science), 2006, 27(3): 300-303. (in Chinese)
[10] YIN Jian-guo. Study on the seeded agglomeration process of supersaturated sodium aluminate liquors and its enhancement [D]. Changsha: Central South University, 2007: 109. (in Chinese)
[11] WATTS H L, UTLEY D W. Volumetric analysis of sodium aluminate solutions [J]. Analytical Chemistry, 1953, 25(6): 864-867.
[12] ZENG Ji-shu, YIN Zhou-lan, ZHANG Ai-ming, QI Gong-wei, CHEN Qi-yuan. Unusual effect of 1,2-octanediol on sodium aluminate solutions leading to inhibition of gibbsite crystallization [J]. Hydrometallurgy, 2008, 90(2/4): 154-160.
[13] WATLING H R, FLEMING S D, van BRONSWIJK W, ROHL A L. Ionic structure in caustic aluminate solutions and the precipitation of gibbsite [J]. Journal of the Chemical Society Dalton Transactions, 1998, 28: 3911-3917.
[14] MOOLENAAR R J, EVANS J C, MCKEEVER L D. The structure of the aluminate ion in solutions at high pH [J]. Journal of Physical Chemistry, 1969, 74(20): 3629-3636.
[15] MA Shu-hua, ZHENG Shi-li, XU Hong-bin, ZHANG Yi. Spectra of sodium aluminate solutions [J]. Trans Nonferrous Met Soc China, 2007, 17(4): 853-857.
[16] LI Jie. Study on the structural characteristics and decomposition mechanism of supersaturated sodium aluminate solution [D]. Changsha: Central South University, 2001: 86-110. (in Chinese)
[17] YIN Jian-guo, CHEN Qi-yuan, YIN Zhou-lan, ZHANG Jiang-feng. Study on the oscillation phenomena of particle size distribution during the seeded agglomeration of sodium aluminate liquors [J]. Light Metals, 2006: 173-176.
Foundation item: Project(20476107) supported by the National Natural Science Foundation of China; Project(2005CB623702) supported by the National Basic Research Program of China
Corresponding author: YIN Zhou-lan; Tel: +86-731-8877364; E-mail: lbl7701951@163.com
DOI: 10.1016/S1003-6326(08)60344-1
(Edited by YUAN Sai-qian)