Influence of aging treatment on corrosion behavior and mechanism of Mg–Y alloys
来源期刊:中南大学学报(英文版)2018年第5期
论文作者:徐宏 张新 蒋三生 任霁萍 高翔宇
文章页码:987 - 1002
Key words:Mg–Y alloy; aging treatment; electrochemical corrosion resistance; corrosion morphology
Abstract: This paper studied the influence of aging treatment on the corrosion behavior and mechanism of Mg–Y alloys with different Y content through corrosion mass loss test, electrochemical test and corrosion morphologies observation. Results show that the peak-aging times of Mg–(0.25, 2.5, 5, 8 and 15) Y alloys at 250 °C were 4, 6, 10, 12 and 16 h. The aging treatment reduced the corrosion resistance of Mg–Y alloys, and the corrosion resistance of Mg–Y alloys became worse with increasing of the aging time. The change magnitude of the open circuit potentials for Mg–(0.25, 2.5)Y alloys was greater than that of Mg–(5, 8 and 15)–Y alloys. The polarization curves of Mg (0.25, 2.5, 5, 8 and 15) Y alloys had the similar shape after aging treatment, and the slopes of the anodic branch were greater than those of the cathodic branches. After aging treatment, the corrosion modes of Mg–0.25Y and Mg–(2.5, 5, 8 and 15) Y alloys were uniform corrosion and pitting corrosion with small local deep corrosion.
Cite this article as: XU Hong, ZHANG Xin, JIANG San-sheng, REN Ji-ping, GAO Xiang-yu. Influence of aging treatment on corrosion behavior and mechanism of Mg–Y alloys [J]. Journal of Central South University, 2018, 25(5): 987–1002. DOI: https://doi.org/10.1007/s11771-018-3799-3.

J. Cent. South Univ. (2018) 25: 987-1002
DOI: https://doi.org/10.1007/s11771-018-3799-3

XU Hong(徐宏)1, ZHANG Xin(张新)2, 3, JIANG San-sheng(蒋三生)4,REN Ji-ping(任霁萍)1, GAO Xiang-yu(高翔宇)5
1. School of Materials Science and Engineering, North University of China, Taiyuan 030051, China;
2. Institute of Thermal Power Generation Technology, China Datang Corporation Science and Technology Research Institute, Beijing 102206, China;
3. State Key Lab for Fabrication & Processing of Non-ferrous Metals, Beijing General Research Institute for Non-ferrous Metals, Beijing 100088, China;
4. School of Mechanical and Electronic Engineering, Beijing Agricultural Vocation College,Beijing 102208, China;
5. Department of Automotive Engineering, Tsinghua University, Beijing 100083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: This paper studied the influence of aging treatment on the corrosion behavior and mechanism of Mg–Y alloys with different Y content through corrosion mass loss test, electrochemical test and corrosion morphologies observation. Results show that the peak-aging times of Mg–(0.25, 2.5, 5, 8 and 15) Y alloys at 250 °C were 4, 6, 10, 12 and 16 h. The aging treatment reduced the corrosion resistance of Mg–Y alloys, and the corrosion resistance of Mg–Y alloys became worse with increasing of the aging time. The change magnitude of the open circuit potentials for Mg–(0.25, 2.5)Y alloys was greater than that of Mg–(5, 8 and 15)–Y alloys. The polarization curves of Mg (0.25, 2.5, 5, 8 and 15) Y alloys had the similar shape after aging treatment, and the slopes of the anodic branch were greater than those of the cathodic branches. After aging treatment, the corrosion modes of Mg–0.25Y and Mg–(2.5, 5, 8 and 15) Y alloys were uniform corrosion and pitting corrosion with small local deep corrosion.
Key words: Mg–Y alloy; aging treatment; electrochemical corrosion resistance; corrosion morphology
Cite this article as: XU Hong, ZHANG Xin, JIANG San-sheng, REN Ji-ping, GAO Xiang-yu. Influence of aging treatment on corrosion behavior and mechanism of Mg–Y alloys [J]. Journal of Central South University, 2018, 25(5): 987–1002. DOI: https://doi.org/10.1007/s11771-018-3799-3.
1 Introduction
The heavy rare earths have the large solid solubility in Mg alloy, and the solid solubility will decrease drastically with the reduction of temperature, so the heavy rare earths has a very good solution and precipitation strengthening effect for Mg alloy. A large over-saturation situation of Mg alloy may be obtained after the homogenizing treatment, which provided good conditions to enhance the aging precipitation [1–3].
The corrosion mechanism, corrosion behavior and protection measures of Mg alloy are studied extensively [4–6], but there are few studies about the influence rules of aging treatment on the corrosion resistance of Mg–RE alloy. On one hand, the aging treatment enables the supersaturated solid solution to decompose and generate a second precipitated phase. The cathode phases with different sizes are distributed in grains and grain boundaries, which can increase the number and area of cathodes and lead to decrease the corrosion resistance of Mg alloy [7]; on the other hand, the precipitation phases with high thermal stability uniformly and dispersedly precipitate from the Mg–RE alloy after the aging treatment.The precipitation phases refine the alloy structure, facilitate the oxide film on the alloy surface to become loose, and reduce the oxidation tendency. The precipitation phases could enhance the strength, ductility, corrosion resistance, abrasion resistance and other properties of Mg alloy [8]. Therefore, the corrosion principle, corrosion mechanism and corrosion model of Mg–RE alloy have not yet been decided, which need to be further studied. It is of great significance to reasonably study the influence rules of aging treatment on the corrosion properties of Mg alloy. The clear study of the influence rules of aging treatment on the corrosion properties of Mg–Y alloy is of fundamental significance for design development of a new type Mg alloy [9].
Based on the study of corrosion behavior of Mg–Y alloy in cast, homogenized and extruded states [10–12] and Mg–5Y–7Gd–1Nd–0.5Zr alloy [13], this paper will study the corrosion mechanism of Mg–(0.25, 2.5, 5, 8 and 15)Y alloys in the different aging treatment process. The influence rules of aging treatment on the corrosion properties of Mg–Y alloy could offer the basic theory and basic data for studying the Mg–Y–X system heat resistant alloys. To improve the microstructure of Mg alloy, an effective way is to increase the corrosion resistance of Mg alloy. This chapter will improve the microstructure of alloy through aging treatment, and analyze the relationship between the strength and microstructure in different aging treatment states and the corrosion resistance.
2 Test materials and methods
2.1 Preparation of test materials
The test alloys were prepared using commercially pure Mg (99.95%) and Y (99.9%) as raw materials. It was melt in a melting furnace and processed into a Φ200 mm×300 mm ingot according to the procedure designed by ZHANG et al [14]. The composition analysis is shown in Table 1.
The Mg–(0.25, 2.5, 5, 8 and 15)Y alloys were homogenized at 535 °C, and the aging treatment times were 2, 2, 2, 6 and 24 h, respectively. The hot extrusion processing of Mg (0.25, 2.5, 5, 8 and 15)Y alloys were as follows: the alloy ingots were heated to 260 °C in the preheating furnace for 2 h, and the preheated ingots were squeezed into the round bars with the diameter of 20 mm (corresponding to the extrusion ratio was 20) in the YH61-500G type horizontal extruder. The extruded round bars would be forced to cool with water at the outlet of the extruder.
Table 1 Chemical composition of Mg–Y alloys in mass fraction

2.2 Test methods
1) Sample preparation
The dimensions of samples were 10 mm×10 mm×10 mm. The sample was polished respectively with 120 #, 500 #, 800 #, 1000 # and 1200 # SiCp sandpapers. When used, the sample was defined with a 10 mm×10 mm working surface with epoxy resin. One end of the electrochemical test sample was connected with copper wire, and the rest other than the working surface (l.00 cm2) was encapsulated with a denture powder, and stored in a dryer after solidification and grinding.
2) Immersion test
The saline water was the NaCl neutral solution with a concentration of 3.5%. The test temperature was (25±2) °C. Samples were continuously immersed in the solution, and taken out every 5 h. The corrosion products on the sample surface were cleaned with 20% CrO3. The change in the mass per unit area during the unit time, i.e. the average corrosion rate, is used to express the corrosion rate v (mg/(cm2·h)), as shown in the following formula [15]:
 (1)
(1)
where A was sample area (cm2); t was test time (h); w0 was original mass of sample (mg); w1 was mass of sample after corrosion products were removed (mg); w2 was correction mass loss of the blank sample with the same size and material after removal of corrosion products.
3) Electrochemical test
The electrochemical testing system, composed of PGSTAT 30 Potentiostat/Galvanostat Model 273A and HF frequency response analyzer SI1255 as well as the appropriate test analysis software, were used to determine the electrochemical impedance spectroscopy of the sample in 3.5% NaCl solution at 25 °C. The polarization curves of the alloy in 0.1 mol/L NaCl aqueous solution were measured under as the following conditions: scanning range from –250 to 125 mV; scanning speed of 0.5 mV/s; initial open-circuit potential of –0.35 V; open-circuit potential in the end of 0.5 V; effective exposure area of 1 cm2; sine wave with excitation signal amplitude of 5 mV; and frequency range of 20000–0.005 Hz.
4) Hardness test
The hardness test was conducted using 430SVD Vickers hardness tester. The sample was ground with 2000# grit sandpaper. The loading force was 30 kg and loading time was 10 s. 10 valid values were obtained from each sample and their average value was taken for determining the final result.
5) Corrosion morphology
The samples were corroded with 4% nitric acid alcohol, and then observed and analyzed in different states using JSM-6510A scanning electron microscope (SEM). After each immersion test, the sample was placed in 400 mL boiled solution containing 10% CrO3 and kept for 5–8 min. Next, clear up the corrosion products and test the corrosion morphology of the sample using the SEM.
3 Results and discussions
3.1 Effect of aging treatment on hardness of Mg–Y alloys
The aging treatments temperatures in this study are 210, 230 and 250 °C. The hardness curves of Mg–(0.25, 2.5, 5, 8 and 15) Y alloys after aging treatment are shown in Figure 1. When the aging temperature is 210 °C, the hardness of Mg–Y alloys increases rapidly during early aging time. When reaching the peak aging, the hardness fluctuates within a small range and basically keeps unchanged as further aging continues, which shows a good thermal stability.
When the aging temperature rises to 230 °C and 250 °C, the hardness of Mg–0.25Y alloy fluctuates within a small range due to low content of Y, but the hardness basically keeps unchanged, which also shows the good thermal stability. As for Mg–(2.5, 5, 8 and 15) Y alloys, the over-aging occurs as a result of further extension of aging time and increasing growth of the precipitated strengthening phase. In this case, the hardness of the alloys rapidly drops to a certain value and fluctuates within a small range with aging continuing. When the aging temperature reaches to 250 °C, the reaching time to the peak aging would be shortened, but the peak aging value will not be significantly reduced. The peak-aging times of Mg–(0.25, 2.5, 5, 8 and 15)Y alloys at 250 °C are 4, 6, 10, 12 and 16 h, respectively. In this study, the aging processes used of Mg–(0.25, 2.5, 5, 8 and 15) Y alloys are (250 °C, 2 h), (250 °C, 10 h) and (250 °C, 24 h), respectively.
3.2 Effect of aging on open-circuit potential of Mg–Y alloys
The Mg–(0.25, 2.5, 5, 8 and 15)Y alloys were treated under different aging processes and immersed in the 3.5% NaCl neutral solution at room temperature for 2000 s, and the changing tendencies of the open circuit potential are shown in Figure 2. When the Y content is less than 2.5%, the open circuit potential would continue to rise along with increasing of Y content. When the Y content is greater than 2.5%, the open-circuit potential would decrease along with increasing of Y content. It could be seen that: 1) When Mg–(5, 8 and 15) Y alloys were immersed in the solution, their self-corrosion potential would almost keep unchanged along with increasing of immersion time, just fluctuating within a small range; 2) When Mg–(0.25 and 2.5) Y alloys were immersed at the early stage, their open-circuit potential would rise fast along with increasing of immersion time. As the test continues, the open circuit potential would decrease, and fluctuates within a small range after reaching a certain value. In addition, the reaching time to the open-circuit potential peak would be significantly shortened with increasing of Y content.

Figure 1 Hardness value of Mg–Y alloys under different aging conditions
The open-circuit potential values of the five alloys were lower to a certain extent than that of the extruded alloy [12], which indicated that the second phase Mg24Y5 has accelerated the corrosion process to the alloy as cathode.
It can be observed that the open-circuit potentials of the five alloys reached to a stable value 1200 s later. Because the Mg alloy itself is high chemical inertness, a layer of oxide film is formed in the atmospheric environment. In the early immersion stage, the corrosion medium continuously permeates into the base interface, and then the film gets damaged, which causes the gradual decrease in the corrosion potential. Meanwhile, as the corrosion of the Mg alloy in the solution belongs to the hydrogen evolution corrosion, the corrosion process may cause the increase in partial pH value, resulting in the generation of insoluble corrosion products, such as Mg(OH)2. The insoluble corrosion products might block the channel for diffusion of the corrosion medium and increase the density of the film so that the open-circuit potential of the alloy gradually increases again.

Figure 2 Effect of aging system on open circuit potential:(1: Mg–0.25Y; 2: Mg–2.5Y; 3: Mg–5Y; 4: Mg–8Y;5: Mg–15Y)
3.3 Effect of aging on polarization curves of Mg–Y alloys
The polarization curves of Mg–(0.25, 2.5, 5, 8 and 15) Y alloys were immersed in the 3.5% NaCl neutral solution at room temperature, and tested after the open circuit potential became stable. The results are shown in Figure 3. The anode branch of Mg–Y alloy polarization curves generally represents the dissolution of α-Mg-based phase,while the cathode branch represents the cathode hydrogen evolution of Mg24Y5 phase. The polarization curves of the five alloys treated by different aging processes are of similar shapes. All the anode currents rise faster. The polarization curves of the five are asymmetrical with each other, and the slope of the anode branch is larger than that of the cathode branch, which indicates that the anode plays a more important role in the corrosion reaction.

Figure 3 Effect of aging system on polarization curve of alloy:(1: Mg–0.25Y; 2: Mg–2.5Y; 3: Mg–5Y; 4: Mg–8Y;5: Mg–15Y)
Based on the change in the polarization curves, there is no passivation behavior in the polarization process of the five alloys after aging treatment, the reason for which is that the Cl– absorbed onto the Mg alloy surface was easy to cause the generation of Mg (OH)2, and Mg (OH)2 would be transformed into the soluble MgCl2. So, no passivation behavior occurred in the 3.5wt% NaCl solution.
In the polarization process, the corrosion current increased rapidly. As observed in the test, the hydrogen evolution rate also significantly increased, indicating that the polarization process in NaCl solution did not comply with the conventional Tafel Law and the corrosion current cannot be calculated using the law. All the anode currents rise fast, indicating serious corrosion of the alloy in 3.5% NaCl solution occurring. Corresponding to the cathodic hydrogen evolution reaction, the cathodic Tafel slope bc of polarization curves of the five alloys varied greatly, indicating that the cathodic reaction course depended on the Y content; while the anode Tafel slope ba was of small difference and the anodic polarization curves of the surface moves to the low current density direction.
The Tafel parameters of Mg–Y alloy are shown in Table 2. There is a negative difference effect in the corrosion of alloy, namely, the cathodic hydrogen evolution also increased with increasing of anodic polarization. So, it is not reliable to calculate the corrosion rate of Mg alloy relying on the traditional polarization curve method, but it may be used to determine the corrosion tendency of Mg alloy.
The negative shift occurred in the corrosion potential of Mg–RE alloy with aging treatment continuing. The anode current density shot up when it reached a certain value, but the anode current densities were of small difference. The positive shift occurred in the anode current density as the aging continued. Besides, the cathode current densities increased to a certain extent along with aging, indicating that both cathode and anode reactions were promoted at the same time, which revealed that the cathode and the anode reactions went fast after aging treatment, and the corrosion resistance was reduced. Besides, the corrosion resistance gradually decreased along with aging treatment continuing. According to the polarization curves under each of the aging processes, anode and cathode current densities showed the increasing tendency with increasing of Y content, and the increasing tendency becomes more obvious with aging treatment continuing.
It can be seen from the changes in the polarization curves that Mg24Y5 was greatly affected by the change tendency of the self-corrosion potential of the alloy. As the aging continues, Mg24Y5 phase would precipitate continuously. The precipitated Mg24Y5 phase of Mg–(0.25 and 2.5) Y alloys, as cathode in corrosion mode, made the self-corrosion potential move in the negative direction, which accelerated the corrosion to the alloy. While the gradually-precipitated Mg24Y5 phases of Mg–(8 and 15) Y alloys formed the corrosion barrier together with the original Mg24Y5 which was not in the solid solution state. The corrosion barrier made the self-corrosion potential move in the positive direction, which reduced the corrosion of the alloy. The change of Mg–15Y was most obvious, indicating that it was determined by the corrosion mechanism in the case of different Y contents. In addition, the movement of the polarization curves was actually due to the difference in the pitting potential (Ept), which just matched the test results of self-corrosion potential.
Table 2 Polarization curve fitting parameters

Due to the negative difference effect of the Mg alloy [16], its polarization behavior is relatively complex, and cannot be expressed by the traditional Tafel formula. Therefore, the results of polarization curves cannot better distinguish the effect of the rare earth element content on the corrosion properties of Mg alloy. In this case, we should use the electrochemical impedance spectroscopy as well as the corrosion rate test to further access the corrosion resistance change of Mg alloy in the follow-up work.
3.4 Effect of aging on impedance spectroscopy of Mg–Y alloys
In the open-circuit potential status, the changing tendencies of the impedance spectroscopy for Mg–(0.25, 2.5, 5, 8 and 15)Y alloys which were treated under different aging systems are shown in Figure 4. It can be shown that the changing tendencies of the impedance spectroscopy for the five alloys were similar to those of the as-cast alloy, namely, the Nyquist plot of the five alloys include three obvious circuits (a high frequency capacitance circuit, an intermediate frequency capacitance circuit and a short low-frequency induction circuit), which indicated that the aging treated and as-cast Mg alloy had the identical corrosion mechanism. It also indicated that the cathode and anode reaction processes are influenced by the coverage and aggregation degree of anode reaction products by the electrode potential. The high frequency capacitance circuit occurred within a high frequency range, corresponding to double-layer charge transfer resistance and film effects. The diameter of the high-frequency capacitance circuit could accurately reflect the charge transfer resistance (Rt) of the active corrosion electrode [17]. The larger the capacitive arc is, the larger the charge transfer resistance of the active corrosion electrode will be. Besides, the capacitive arc also reflected the capacitance value of the electrochemical double electric layer. The high frequency capacitance circuit was attributed to the charge transfer reaction of the double electric layer forming between the alloy interface and the corrosion medium. The intermediate frequency capacitor circuit appeared within the mid-frequency range, and it is not relevant to the surface film forming on the surface of the test sample. The intermediate frequency capacitor circuit was derived from the diffusion of the porous surface film of the alloy. According to the test results of the polarization curve, it is found that there is a layer of oxidation film on the alloy surface. The low-frequency induction circuit occurs within the low frequency range, and it is relevant to the hydrogen corrosion process of the alloy, and the low-frequency induction circuit was derived from the pitting corrosion nucleation of the initiation process.
As seen from Figure 4, the high-frequency arc radius after aging treatment became smaller compared to the radius of the extruded alloy, but still larger than the high-frequency arc radius of the cast alloy, which indicated that the corrosion resistance after aging treatment decreased in a certain extent compared to the extruded alloy, but still higher than the as-cast alloy [10–12]. As for the effect of Y on the impedance spectroscopy, the high-frequency capacitive arc radius is significantly increased when the Y content increased, and the high-frequency capacitive arc of Mg–5Y alloy increased more significantly than that of Mg–0.25Y. When the Y content reached to 2.5%, the high-frequency capacitive arc radius was the largest. Thereafter, Y content further increased, but the high-frequency capacitive arc radius decreased instead. The larger the high frequency capacitive arc radius is, the larger the resistance value of the corrosion film on the alloy surface will be, and the better the protection property of the corrosion film layer will be, then the corrosion resistance was improved. Therefore, the high-frequency capacitance ring of Mg–Y alloy significantly increased with increasing of Y content, which indicated that the corrosion resistance was improved. When the Y content exceeds 5%, the high-frequency capacitance arc would decrease, which indicated that the corrosion resistance slightly reduced. This test result was consistent with the variation law of the weight loss corrosion rate. The high frequency capacitive arc was mainly caused by the charge transfer effect and the corrosion layer on the alloy surface. So, adding Y into Mg–Y alloy has increased the corrosion film layer on the alloy surface and improved the protection property of the corrosion film. Therefore, the high-frequency capacitive arc of the alloy significantly would increase after adding Y element, and so does the impedance value of the alloy corrosion film layer. When Y content continues to increase, a number of second phases Mg24Y5 would form, which would accelerate the corrosion to the alloy. However, when the Y content further increased, the second phase Mg24Y5 would form the net-like phase as the corrosion barrier and inhibit the corrosion to the alloy to a certain extent.

Figure 4 Effect of aging system on impedance spectra of alloy:(1: Mg–2.5Y; 2: Mg–8Y; 3: Mg–15Y; 4: Mg–8Y; 5: Mg–0.25Y)
The high-frequency arc radius of the five alloys will decrease with aging treatment continuing, which indicated that the corrosion- resistance property would decrease with increasing of the aging time, the reason for which was that the precipitated Mg24Y5 phase accelerates the corrosion in the aging process.
Within the low frequency range of impedance spectroscopy, the alloys show significant inductance behaviors, and the negative values appear at some points. Therefore, the diameter of the low frequency arc was significantly smaller than that of the high frequency arc. Within the high-frequency arc range, the test results were not regularly distributed, the reason for which was that the oxidation film forming on the Mg alloy surface and the corrosion product film forming in the early test stage are not uniform.
The phase angle of Mg–Y alloy was more than 45°, and the maximum phase angle of the high- frequency arc exceed 70°. According to |Z| versus frequency curve in the Pourbaix diagram, the |Z| increase law of Mg–Y alloy was as follows: Mg–0.25Y As observed in the Bode diagram [18], the phase angle decreased with increasing of Y content within the entire test frequency range, which indicated that the strong pitting corrosion feature of the alloy in the electrode surface activation process gradually increased. However, once the corrosion product layer was generated, the impedance modulus would gradually increase, and the capability to prevent ions from invading gradually enhanced, thus effectively suppressing the pitting process. Judged on this basis, the corrosion product layer of the alloy would become more dense and effective along with increasing of Y content. The Nyquist diagram for the early corrosion was the inductive arc in the low frequency region, which was mainly because there was a layer of oxidation film on the alloy surface and the film would be damaged with increasing of the corrosion time. When the oxidation film was damaged, the reaction would occur on the alloy surface, so the inductive arc would become the capacitive arc. According to the corrosion mechanism and corrosion morphology, and based on the electrochemical corrosion principle, the equivalent circuit is shown in Figure 5, where Rs is the solution resistance, Rt is the charge transfer resistance, CPE is the constant phase angle element, Rf is the film resistance and L1 refers to inductance. The impedance spectrum curves of Mg–(0.25, 2.5, 5 and 8) alloys were simulated according to the equivalent circuit. The parameters of the circuit components are shown in Table 3. Figure 5 Equivalent circuit According to the analysis results of the Nyqiust curve equivalent circuit, the sum of the charge transfer resistance Rt and the surface corrosion film resistance Rf of the double electric layer forming on the interface between the high frequency capacitive arc surface and the corrosion medium for Mg–2.5Y alloy is the largest among the five alloys. This means that the corrosion film of this alloy has the largest impedance value, so the corrosion film layer could produce the best effect of protection. When the Y content was lower or higher than 2.5%, a difference would occur between the Y content in the film layer and the proportion of the α-phase, resulting in the poor density of the film layer, so it cannot provide the effective protection to the base metal. At the same aging temperature, the second phase precipitated from the solid solution with the extension of the aging time and the protection property of the film layer forming on the alloy surface decrease, namely, the corrosion resistance decreases. Besides, the ascending order of the corrosion resistances of the five alloys after aging was as follows: Mg–0.25Y Table 3 Impedance spectra fitting parameters of different aging systems 3.5 Effect of aging on corrosion rate of Mg–Y alloys After the aged sample immersing in the solution, the bubbles appeared on the surface of each alloy. With the extension of the aging time, the number of bubbles gradually increased, and the solution got turbid slowly [19]. The changing curves about the effects of aging on the corrosion rate are shown in Figure 6. The aged sample had the increased galvanic corrosion due to the precipitation of the second phase, so the corrosion resistance of the alloy was weakened. With the extension of the aging time, the corrosion resistance of the alloy would also be weakened. It can be seen that the corrosion resistance of the sample which has been subject to 8 h homogenizing treatment was weaker than that of the sample which has been subject to 16 h homogenizing treatment. The reason for that was that the β phase increased with the precipitation in the homogenizing process, thus reducing the corrosion resistance of the alloy to a certain extent. Compared with the extruded alloy, the corrosion rate of aged alloys was reduced at a certain degree, and it increased with the increase of the aging time, so it was concluded that the aging treatment reduced the corrosion resistance of the alloy. By comparing the overall corrosion rate diagrams of the five alloys, it is concluded that the changing tendencies of corrosion rates for Mg–Y series alloys are similar. Effects of phase precipitation on the corrosion of the alloy are analyzed from the microscopic view: the anode phase with more-negative potential, i.e. α-Mg phase and the cathode phase with more positive potential, namely β-Mg24Y5 phase forms the galvanic corrosion pair. Assume that only the reduction reaction of hydrogen occurs on the β phase after entering NaCl solution, and ignore its anodic dissolution current I2,α. According to the mixed potential theory, the total hydrogen evolution current of the two metals is equal to the total reduction current under galvanic potential Eg, namely [20]: where I1,α was anode dissolution current of α phase in galvanic pair; I1,c and I2,c were reduction currents of α and β phases respectively. The cathode is controlled by the hydrogen evolution, so the cathode current densities are equal, i.e. the limit hydrogen evolution current density, namely: Import Formula (2) into Formula (3), and we will obtain: Galvanic current: Namely, Thus, it can be seen that the galvanic current and the cathode-anode area ratio are proportional. The smaller the cathode-anode area ratio is, the smaller the galvanic corrosion current will be, and the slighter the galvanic corrosion will be as well. Therefore, the precipitation of the β phase after the aging treatment increases the cathode-anode area ratio, so does the corrosion current density of the alloy. It means that the aging treatment reduces the corrosion resistance of the alloy, which just coincides with the corrosion mass loss rate of the alloy [21]. Figure 6 Effect of aging on corrosion rate:(1: Mg–0.25Y; 2: Mg–8Y; 3: Mg–15Y; 4: Mg–5Y; 5: Mg–2.5Y) 3.6 Effect of aging on corrosion morphology of Mg–Y alloys The corrosion morphologies of Mg–(0.25, 2.5, 5, 8 and 15)Y alloys, which were respectively aged with (250 °C, 1 h), (250 °C, 10 h) and (250 °C, 24 h) and immersed in 3.5% NaCl solution for 2 h, are shown in Figure 7. When the alloy was immersed in 3.5% NaCl neutral solution, the bubbles quickly attached onto the surface of each alloy. Within 5 to 10 min, the bubbles precipitated on each surface, and the significant pitting began to occur, corrosion damage appeared in partial areas of the alloy sample and some surface layer scaled off. With increasing of the immersion time, the pitting gradually increased, and the corrosion damage area was expanding. Before immersion, the alloy surface was covered with a layer of protective oxidation film, which resulted in a large charge transfer resistance in the initial reaction. After immersing for some time, the chloride ion erosion damaged the oxidation film, and the protection property was reduced. After immersing for 2 h, the corrosion form varied due to different components. The surface layers of Mg–(0.25 and 2.5) Y alloys might scale off, and the corrosion morphologies were the uniform corrosion. However, what is different in this alloy compared to the as-cast alloy and the alloy subject to homogenizing treatment includes: after aging, the phase containing rich Y began to precipitate at the grain boundary. During occurrence of the uniform corrosion,the Y content decreased, and the intergranular phase corrosion resistance was weaker than grains, which resulted in the fact that the intergranular corrosion morphology became obvious. In addition, the intergranular corrosion was significantly increased, which indicates that the phases containing rich Y increased at the grain boundary. The corrosion cathode accelerated the corrosion to the adjacent α-Mg during the formation of uniform corrosion. The corrosion scaling of α-Mg caused the scaling of the phases containing rich Y, thus forming the intergranular corrosion characteristic morphology [22]. The SEM images of alloys with different compositions and different corrosion time after corrosion, are shown in Figure 8. Figure 7 Effect of aging time on corrosion morphology For Mg–(5, 8 and 15) Y alloys, the corrosion morphologies were still pitting and partial. After aging, the flowing line of the corrosion morphology in the alloy's extruded section gradually became clear with increasing of Y content. When the Y content reached to 15%, the corrosion flowing line was more obvious, indicating many Mg24Y5 phases precipitating. Mg24Y5 phases made the corrosion characteristic more obvious on the basis of original corrosion of the extruded alloy. As the aging was going on, the corrosion was accelerated, and the corrosion resistance decreased. Figure 8 SEM images of alloys with different compositions and different corrosion times It can also be seen that when the Y content increases, the degree of corrosion would decrease. When the Y content was 2.5%, the alloy will have the largest corrosion resistance. When the Y content is further increased, the corrosion would be even more serious. Figure 9 Schematic diagram of corrosion process at grain boundary The corrosion products generated from the reaction continuously deposit on the surface of the alloy. The corrosion product film became thickening with aging treatment continuing. When it reached to a certain thickness, the upper-layer corrosion products and the corrosion products continuously generated on the lower layer caused the generation of the stress in the film layer. Meanwhile, the volume change in the film layer relative to the base would also caused the generation of stress at the boundary between the film and the base, and finally caused cracking of the film layer, which provided the Cl– diffusion channel for the continuous erosion. So, the corrosion was further expanded to the internal part of the base. Therefore, the parts with cracks or gaps were more seriously corroded than other parts. Figure 9 is the enlarged view of the non-significantly-corroded area of the corrosion morphologies on the alloy surface (see Figures 7 and 8). It can be seen that the corrosion of the alloy after aging was dissolved in partial area, especially the corrosion dissolution occurring at the grain boundary resulted in holes and cracks of the material. With the corrosion dissolution going on, the corrosion pits continuously extend along the grain boundary and form the partial corrosion product deposits. 4 Conclusions 1) The alloy has significant aging hardening characteristics, and the peak value of its aging hardening curve appears at 210 °C, 230 °C and 250 °C. The hardness of Mg–0.25Y alloy fluctuates within a small range due to a small content of Y and basically keeps unchanged; while the hardness values of Mg–(2.5, 5, 8 and 15) Y alloys first decrease to a certain value quickly after reaching the peak aging as the precipitated strengthening phases grow more quickly, extending the aging time and causing overaging. And then, the hardness fluctuates within a small range. And it would not further decrease with the extension of the aging time. 2) The peak value will decrease with the increase in temperature. Besides, the time required to reach the peak value is shortened. For Mg–(0.25, 2.5, 5, 8 and 15) Y alloys, the time that is required to reach the peak value at 250 °C is 4, 6, 10, 12 and 16 h, respectively. 3) After aging, the open-circuit potential and the self-corrosion potential of the same alloy decrease to a certain extent relative to the extruded alloy. In addition, they further decrease as the aging is going on, indicating that the aging decreases the corrosion resistance of the alloy, and the longer the aging time is, the weaker the corrosion resistance of the alloy will be. 4) After aging, the polarization curves of Mg–(0.25, 2.5, 5, 8 and 15) alloys are of the similar shapes. All the anode currents rise faster and are asymmetrical with each other. The slope of the cathode branch is larger, indicating the cathode plays a more important role in the corrosion reaction. Besides, no passivation phenomenon is observed in the polarization process. 5) In contrast to the extruded alloy, the aged alloy had a reduced high frequency capacitive arc, and its capacitive radius will decrease with the increase of the aging time, indicating that the corrosion resistance of the alloy decreases as the aging goes on. 6) The corrosion mechanism of the alloy after agingis that, the corrosion morphology of Mg–0.25Y alloy is the uniform and the corrosion morphologies of Mg– (2.5, 5, 8 and 15) Y alloys are of pitting corrosion and small local deep corrosion. References [1] SONG G, ATRENS A, DARGUSCH M. Influence of microstructure on the corrosion of die cast AZ80D [J]. Corrosion Science, 1999, 41(5): 249–273. [2] APPS P J, KARIMZADEB H, KING J F, LOTIMER G W. Phase compositions in magnesium-rare earth alloys containing yttrium, gadolinium or dysprosium [J]. Scripta Materialia, 2003, 48(5): 475–481. [3] WANG Jun, MENG Jian, ZHANG De-ping, TANG Ding-xiang. Effect of Y for enhanced age hardening response and mechanical properties of Mg-Gd-Y-Zr alloys [J]. Materials Science and Engineering A, 2007, 456(1, 2): 78–84. [4] ATRENS A, SONG G L, CAO F, SHI Z, BOWEN P K. Advances in Mg corrosion and research suggestions [J]. Journal of Magnesium and Alloys, 2013, 1(3): 177–200. [5] WEN Li, YU Kun, FANG Hong-jie, XIONG Han-qing, YIN Xiang, ZHU Hua-long, MA Jia-ji, JIANG Da-yue. Electrochemical behavior of Mg-Al-Pb alloy in 3.5% NaCl solution [J]. Journal of Central South University, 2016, 23(10): 2475–2482. [6] SHI Z, LIU M, ATRENS A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation [J].Corrosion Science,2010, 52(2): 579–588. [7] NIE J F, MUDDLE B C. Characterisation of strengthening precipitate phases in a Mg–Y–Nd alloy [J]. Acta Materialia, 2000, 48(8): 1691–1703. [8] HONMA T, KAMADO S, HONO K. Effect of Zn additions on the age-hardening of Mg-2.0Gd-1.2Y-0.2Zr alloys [J]. Acta Materialia, 2007, 55: 4137–150. [9] MA Ming-long, ZHANG Kui, LI Xing-gang. The hot deformation behavior of as-cast Mg-7Gd-5Y-1.2Nd-Zr magnesium alloy [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(s1): 132–139. [10] ZHANG Xin, ZHANG Kui, DENG Xia, LI Hong-wei, LI Yong-jun, MA Ming-long, LI Ning, WANG Yan-long. Corrosion behavior of Mg-Y alloy in NaCl aqueous solution [J]. Progress in Natural Science: Materials International, 2012, 22(1): 169–174. [11] ZHANG Xin, LI Yong-jun, ZHANG Kui, WANG Chang-shun, LI Hong-wei, MA Ming-long, ZHANG Bao- dong. Corrosion and electrochemical behavior of Mg-Y alloy in 3.5wt.% NaCl solution [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1226–1236. [12] XU Hong, ZHANG Xin, ZHANG Kui. Effect of Y extrusion on corrosion behavior and corrosion mechanism of Mg–Y alloy [J]. Journal of Rare Earths, 2016, 34(3): 315–327. [13] ZHANG Xin, ZHANG Kui, LI Xing-gang, DENG Xia, LI Hong-wei, ZHANG Bao-dong, WANG Chang-shun. Comparative study on the corrosion behaviour of as-cast and extruded Mg-5Y-7Gd-1Nd-0.5Zr magnesium alloys in 5wt.% NaCl aqueous solution [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(5): 1018–1027. [14] ZHANG Kui, LI Xing-gang, LI Yong-jun. Effects of Gd contents on microstructure and mechanical properties of Mg-Y-RE-Zr magnesium alloy [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(s1): 12–15. [15] ALISON J D, CRISIANO P, BRIAN J C, NICHOLAS S, THOMAS A W B, AMELA G, MACRO S. Synchrotron X-ray microtomography study of the role of Y in corrosion of magnesium alloy WE43 [J]. Electrochem Solid-State Lett, 2007, 10: C5–C8. [16] SONG G, BOWLES A L, STJOHN D H. Corrosion resistance of aged die cast magnesium alloy AZ91D [J]. Materials Science and Engineering A, 2004, 366(1): 74–86. [17] MATHIEU S, RAPIN C, HAZANL J. Corrosion behavior of high pressure Di-cast and semi-solid cast AZ91D alloys [J]. Corrosion Science, 2002, 44: 2737–2756. [18] SONG G. Recent progress in corrosion and protection of magnesium alloys [J]. Advance Engineering Materials, 2005, 7(7): 563–586. [19] ZHANG Jing-hai, NIU Xiao-dong, QIU Xin. Effect of yttrium-rich misch metal on the microstructures, mechanical properties and corrosion behavior of die cast AZ91 alloy [J]. Journal of Alloys Compounds, 2009, 471(1): 322–330. [20] HE Wei-wei, ZHANG Er-lin, YANG Ke. Effect of Y on the bio-corrosion behavior of extruded Mg-Zn-Mn alloy in Hank’s solution [J]. Materials Science and Engineering C, 2010, 30(1): 167–174. [21] SHI Zhi-ming, LIU Ming, ATRENS A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation [J]. Corrosion Science, 2010, 52(2): 579–588. [22] WU Guo-hua, FAN Yu, GAO Hang-tao, ZHU Yan-ping. The effect of Ca and rare earth elements on the microstructure, mechanical properties and corrosion behavior of AZ91D [J]. Materials Science and Engineering A, 2005, 408(1, 2): 255–263. (Edited by YANG Hua) 中文导读 时效处理对Mg–Y合金腐蚀行为和腐蚀机理的影响 摘要:本文采用腐蚀失重试验、电化学测试和腐蚀形貌观察等研究方式研究了时效处理对不同Y含量Mg–Y合金的腐蚀行为和腐蚀机理的影响机制。研究结果表明:Mg–(0.25,2.5,5,8,15)Y合金在250 °C时的峰时效时间分别为4、6、10、12和16 h;时效处理降低了Mg–Y合金的耐腐蚀性能,并且对于同一种Mg–Y合金的耐蚀性能随着失效时间的延长而逐渐降低;Mg–(0.25, 2.5)Y合金的开路电位的变化幅度远大于Mg–(5,8,15) Y合金的开路电位的变化幅度;时效处理后,Mg–(0.25,2.5,5,8,15)Y这5种合金有着相类似的极化曲线形状,并且阳极曲线的斜率均大于阴极曲线的斜率;时效处理后,Mg–0.25Y合金的腐蚀机理为均匀腐蚀,而Mg–(2.5,5,8,15)Y合金的腐蚀机理为局部腐蚀较深的点蚀。 关键词:Mg–Y合金;时效处理;电化学腐蚀;腐蚀形貌 Foundation item: Projects(2011BAE22B01, 2011BAE22B06) supported by the National Key Technology R&D Program, China Received date: 2016-09-12; Accepted date: 2016-12-08 Corresponding author: XU Hong, PhD, Professor; Tel: +86–351–3922012; E-mail: xh725@263.net

 (2)
(2) (3)
(3) (4)
(4)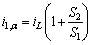 (5)
(5)
 (6)
(6) (7)
(7)




