J. Cent. South Univ. Technol. (2007)05-0666-07
DOI: 10.1007/s11771-007-0128-7 
Synthesis and properties of acrylate latex modified by vinyl alkoxy siloxane
ZHANG Xin-ya(张心亚), SUN Zhi-juan(孙志娟), HUANG Hong(黄 洪),
LI Yong-jin(黎永津), LAN Ren-hua(蓝仁华), CHEN Huan-qin(陈焕钦)
(School of Chemical and Energy Engineering, South China University of Technology, Guangzhou 510641 , China)
Abstract: Acrylate latex modified by vinyl triisopropoxy silane (C-1706) was synthesized by seeded emulsion polymerization with anionic emulsifier sodium dodecyl sulphonate(SDS) and nonionic emulsifier OP-10 as the multiple emulsifiers at (78±2)℃. The effects of different factors, such as the emulsifier, C-1706 monomer and its feeding manner on the properties of acrylate latex modified by C-1706 were investigated. The particle size distribution and the structure, the configuration, the weather durability and stain resistance of copolymer latex were characterized by particle size analyzer, Fourier transform infrared spectroscopy (FT-IR), transmission electron microscope(TEM), scanning electron microscope(SEM) and ultraviolet aging instrument respectively. The results show that SDS to OP-10 as multiple emulsifiers can lead to coordinated efficiency, the optimal emulsifier dosage is 2.4%-3.2%(mass fraction), and the mass ratio of SDS to OP-10 is 1?1- 1?2. The seeded emulsion polymerization can effectively introduce a organic-siloxane bonding in a macromolecule inter polymer, and the obtained acrylate latex modified by organic-siloxane possesses narrow distribution of particle size with mean diameter of 51.8-76.6 nm and has the excellent properties in weather durability and stain-resistance especially.
Key words: vinyl alkoxy siloxane; acrylate latex; emulsion polymerization; synthesis; characterization
1 Introduction
Vinyl alkoxy siloxanes have widespread application as functional monomers in the synthesis of acrylate polymers for both solventborne and waterborne coatings. These monomers as adhesion promoters and latent crosslinkers improved the physical and chemical properties such as adhesion, solvent resistance, abrasion resistance, and especially weather durability and stain resistance[1-3]. The more level of incorporation of the vinyl alkoxy siloxane monomer is, and the better the properties are. A number of techniques have been developed for the preparation of acrylate polymer latexes system modified by vinyl alkoxy siloxane[4-7].
Meanwhile there are some inherent pitfalls in using vinyl alkoxy siloxanes for waterborne systems[4-7]. For example, if the initial vinyl alkoxy siloxane concentration in water phase is too high, the hydrolyzable groups in vinyl alkoxy siloxane will react with water, subsequently self-condensing to form siloxane crosslinks with a network in the pot[8-9]. Extremes in pH value accelerate the hydrolysis and crosslinking. Premature gelation, viscosity buildup and poor final coating performance are all possible results from the vinyl alkoxy siloxane hydrolysis and condensation[10]. Thus, these vinyl alkoxy siloxanes are limited in the levels in a one-pack system. Often, good properties that could theoretically be achieved from incorporation of higher levels of siloxane are not achieved due to the reactivity and short pot life of the added vinyl alkoxy siloxanes[11-12]. In this paper, seeded emulsion polymerization of all-acrylate in the presence of a vinyl triisoproxy silane (C-1706) was studied. The C-1706 monomer has more bulky isopropoxy substituents, allowing them to restrain the hydrolysis of the hydrolyzable groups efficiently and enhance the stability of the emulsion polymerization process. And then, the analytical techniques including FT-IR, SEM, TEM, UV and particle size analyzer were used to characterize the properties and structures of the copolymer latex.
2 Experimental
2.1 Materials
The monomers of methyl methacrylate (MMA), butyl acrylate (BA) and methacrylic acid (MAA) were industrial grade available in China. Vinyl triisopropoxy silane (C-1706) was produced by Crompton Business(America). Potassium perosulphate (KPS), sodium bicarbonate (SBC), sodium dodecyl sulphonate (SDS), nonyl phenol ethoxylates (OP-10), deionized water and ammonium hydroxide were chemical grade available in China. The formulation of sample is shown in Table 1.
Table 1 Formulation of sample

2.2 Preparation of all-acrylate latex modified by C-1706
Seeded emulsion polymerization of all-acrylate latex modified by C-1706 was carried out in a 2 L four- neck glass flask equipped with a stirrer, a reflux condenser, a thermometer, and two feeding inlets. The procedure is as follows.
1) Charge the emulsifiers and de-ionized water into the flask and dissolve the emulsifiers with gentle stirring. Set the circulator temperature controller to maintain the initial charge at 78 ℃.
2) Mix monomers with the predetermined ratio.
3) Once the initial reaction temperature reaches 78 ℃, add 20%(mass fraction) KPS solution and 10%(mass fraction) monomer mixture via two separate feeding inlets in about 15 min. Let the reaction run further for 15 min at (78±2)℃ with strong agitation.
4) Start to feed the rest of monomer mixture and KPS solution through two separate feeding inlets when the emulsion is bluish. The feeding rates should be adjusted to make the feeding complete in about 4 h. Maintain the reaction temperature at (78±2)℃ during the whole feeding.
5) Hold the reaction at (78±2) ℃ for 30 min and further raise the reaction temperature to 85 ℃ for another 30 min after the monomer mixture and KPS solution have been feed completely.
6) Cool the resulted latex to 40 ℃, adjust pH value to about 7.5 using ammonium hydroxide solution and filter the latex.
2.3 Characterization of reaction product
The viscosity was tested by a Brookfield RVDL-II+ viscosimeter with 2# rotor of 60 r/min at 25 ℃.
The methyl ethyl ketone(MEK) resistance was tested according to ASTM D5402.
The stability of emulsion polymerization can be denoted by agglomeration ratio η. Collect all agglomerate after emulsion polymerization, drying to constant in the oven and calculate the agglomeration ratio as follows:
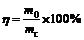 (1)
(1)
where m0 is the initial mass of agglomerate, mc is the final mass of agglomerate after drying.
An accurately weighed sample of coating was placed in a fine wire cage in a Soxhlet extractor, and extracted with refluxing MEK for 8 h. The mass loss of the coating sample was measured accurately with the gel content w(gel) according to:
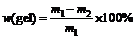 (2)
(2)
where m1 is the initial mass of coating sample, m2 is the final mass of coating sample after extraction.
Soak film sample in MEK for 24 h, and then measure the change of length in situ. The swell ratio was calculated according to the ratio of final to initial values.
The thaw-freezing stability was tested as follows: charge the latex sample into a 500 mL bottle, freeze the sample bottle at (-18±1) ℃ for 18 h and thaw at 20 ℃ for 6 h, measure the variations of the mass of gel and the viscosity of latex during the thaw-freezing process.
The electrolyte stability was tested as follows: prepare 5%Ca2+ solution, mix the Ca2+ solution and the latex sample with the mass ratio of 4:1, observe the variations after placed for 48 h.
A Perkin Elmer 2000 FTIR spectrophotometer equipped with a K Br transmission cuvette was used to measure the spectra for the latex.
The particle size was measured by a Coulter LS 230 particle analyzer.
The morphology of the latex film was studied using a Hitachi S-530 SEM and a JEM-1010.
The ageing properties of the latex film were studied using QUV-66.
3 Results and discussion
3.1 Influence of emulsifier
The type and concentration of emulsifiers are the key factors in the seeded emulsion polymerization. Although the emulsifiers don’t participate in the reaction directly, their type and concentration have very important influences on the initiating rate, the forming and transferring speed of the chain, and the relative molecular mass of the polymer, which affect the latex particles size and their distribution[13]. The experimental results show that the reaction temperature has an important effect on the emulsion polymerization stability when only the nonionic emulsifier is presented. While the latex possesses excellent machine shear stability and worse electrolytical stability when only anionic emulsifier is presented. Thus in this experiment, the anionic emulsifier SDS and the nonionic emulsifier OP-10 were used as multiple emulsifiers.
3.1.1 Influence of amount of emulsifiers
The emulsifier concentration affects not only the forming number of emulsification micelle, but also the resulted latex particle size and viscosity. The influence of the emulsifier concentration on the latex properties is represented in Table 2.
Table 2 Influence of emulsifier concentration on polymer latex

From Table 2, with the emulsifier concentration increasing, the micelle number increases, the initiating and reaction rates are also faster, so the latex particle size decreases. Consequently, the total specific surface area and the mutual forcing between the particles both increase, that is to say, the flowing-resistance of particles also increases, which leads to the latex viscosity increase and the polymerizing process more stable. In order to diffuse the reaction heat produced in time, the latex viscosity should remain moderate. Comprehensively considering the system stability and the latex properties, the preferable emulsifier concentration is within 2.4%- 3.2%.
3.1.2 Influence of mass ratio of anionic to nonionic emulsifier
Anionic emulsifier is used widely in emulsion polymerization. For a nonionic emulsifier, the surface of latex particles has lots of big molecules, which form an absorption layer with certain thickness and intensity to
hinder the cohesion between the latex particles and improve the chemical stability[14]. When the emulsifier concentration is 3.0%, the effects of different mass ratios of SDS to OP-10 are shown in Table 3.
Table 3 Influence of emulsifier ration on properties of copolymer latex
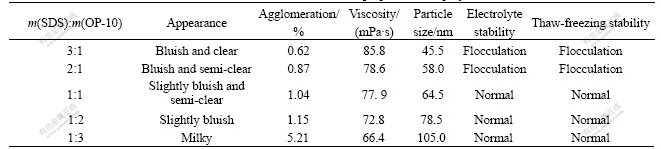
From Table 3, the bluish latex appearance comes forth with the increasing mass ratio of SDS to OP-10 in the polymerization process, but the latex viscosity increases and the electrolyte stability and thaw-freezing stability become worse obviously. When SDS and OP-10 cooperate in harmony with the appropriate ratio, the molecules of SDS wedge into those of OP-10, the distance between the molecules becomes far and the static tension on their surface reduces, so the chemical and thaw-freezing stabilities are improved. The suitable mass ratio of SDS to OP-10 is within 1?1-1?2.
3.2 Influence of alkoxy siloxane monomer
The special structure of vinyl triisopropoxy silane molecular chain endows the excellent properties to the modified acrylate latex. During the film-forming process, the alkoxy siloxane groups in the polymer chain hydrolyze to Si—OH, bonding to a filler or substrate and forming a strong cross-linked Si—O—Si chain. The chemical bond of forming molecular network replaces the molecules forcing, which enhances not only the polymer rigidity, but also the polymer cohesion.
The influence of incorporation of the siloxane on solvent resistance is shown in Fig.1. The all-acrylate latex does not exhibit any solvent resistance, and is immediately dissolved by MEK or acetone. Even incorporation of low levels of siloxane monomer makes a profound difference in terms of the physical and chemical properties. The more the siloxane incorporated, the greater the solvent resistance(see Fig.1).
Gel content and swell ratio are non-mechanical means of determining the crosslinking of the system. Gel content is a measure of the non-extractable insoluble portion of the polymer, while swell ratio is a measure of how “tight” the crosslinking in a given system is. Fig.2 shows the gel content and swell ratio of the latexes modified by C-1706. From Fig.2, it is clear that the higher the content of siloxane in the backbone, the higher the degree of crosslinking.
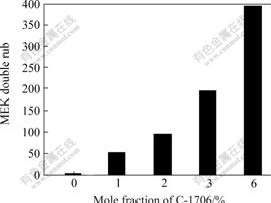
Fig.1 Solvent resistance of acrylate latex modified by C-1706
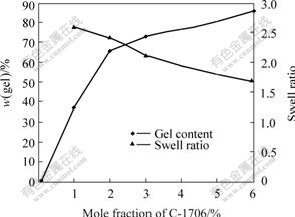
Fig.2 Gel content and swell ratio of latexes modified by C-1706
This highlights the utility of the isopropoxy substituted siloxanes, the enhanced stability under the conditions of the polymerization allows much higher levels of incorporation and better properties polymers. In the case of the vinyl triisopropoxy silane, this higher performance can be seen all the way up to a level of 6%(mole fraction).
3.3 Influence of carboxyl functional monomer MAA
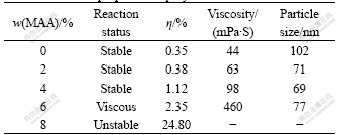
MAA monomer containing carboxyl has an important influence on the particle size and thickening behaviour of acrylate latex. The effect of content of MAA in emulsion formulation on thickening behaviour is shown in Table 4. From Table 4, the particle size decreases obviously and distributes widely with the increasing percentage of MAA. The reason is that MAA has a big distribution coefficient in MMA and water, the water-soluble monomer increases, and results some new no core particle with small particle size inevitable. So the resulted latex particle size becomes rather less and its distribution becomes rather wider. Further, the polymer latex agglomeration ratio and the viscosity increase with the increasing percentage of MAA.
Table 4 Influence of MAA content in emulsion formulation on properties of polymer latex
3.4 FT-IR analysis of polymer structure
The FTIR spectra of the acrylate latex and the C-1706 modified acrylate latex prepared with MMA, BA and MAA are shown in Fig.3. Fig.3 shows that the frames of the two curves are alike. In spectrum of acrylate latex, the hydroxyl absorption is dominated by a band at 3 444 cm-1, the C=O bond of the acrylate polymers stretches around 1 731 cm-1 , the absorbance bands of MAA at 1 239, 1 172, and 1 067 cm-1, and the bands at 990 and 963 cm-1 belong to BA. Comparing spectrum 1 to 2, the accentuated Si—OH at 902 cm-1 shows that some C-1706 monomers have undergone hydrolysis in the polymerization. On the other hand, the characteristic Si—O—Si absorption of C-1706 appears at 1 078 cm-1, and the band of 1 120 cm-1 is characteristic of isopropoxy siloxane. These differences show that C-1706 has copolymerized with acrylate monomers.
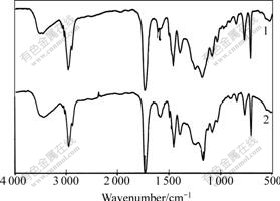
Fig.3 FTIR spectra of acrylate polymer and acrylate copolymer latex modified by C-1706
1—Acrylate latex;2—Acrylate latex modified by C-1706
3.5 Analysis of particle size and distribution
3.5.1 Influence of C-1706
The influence of C-1706 on the polymer latex particle size and its distribution are shown in Fig.4. The particle size of the acrylate latex without C-1706 presented is 51.8 nm and its disperse index is 0.029 7; the particle size is 76.6 nm and its disperse index is 0.0746 when the content of C-1706 is 6%. The results show that with the content of C-1706 increasing, both the particle size and its disperse index increase.
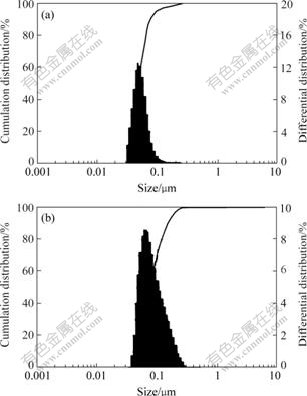
Fig.4 Particle size and distribution of acrylate latex modified by C-1706 w(C-1706)/%: (a) 0; (b) 6
3.5.2 Influence of feeding time of C-1706
The influence of C-1706 feeding time on the latex particle size and its distribution are shown in Fig.5. Because the hydrolyzable alkoxy groups on C-1706 are inherently water sensitive, the added C-1706 content is 3%. From Fig.5, it shows that the latex particle size becomes smaller and its distribution becomes narrower. The seeded emulsion polymerization reaction was carried out under the slightly acidic condition. If the C-1706 feeding into the system too early, the alkoxy groups hydrolyze and condensate in the initial stage, which makes the particles cohere with each other, and thus the particle number decreases, the particle size increases and its distribution becomes wider. The retarding of the C-1706 feeding time can effectively avoid the hydrolysis and polycondensation of alkoxy groups, and the increase of particle size of latex.
3.6 TEM analysis of particle configuration
TEM images of the C-1706 modified acrylate latex particle are shown in Fig.6. It shows that the latex particle configuration of the retarding of the C-1706 feeding is smoother than that of the C-1706 feeding before the reaction, and the particle size is more uniform and smaller. The result is completely coincided with that by the particle size analyzer as above. Besides the great mass of the smooth particles with rather large size, some little size particles are also presented in the TEM images. This is attributed to the particle nucleation in the whole process with vinyl alkoxy siloxane copolymerization, some new anuclear particles are created constantly, which leads to the C-1706 modified latex particle size uneven.
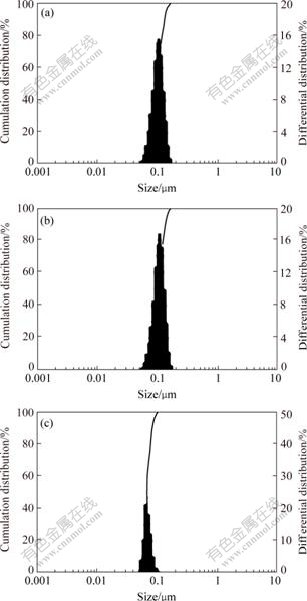
Fig.5 Effects of feeding time of C-1706 on particle size and distribution of acrylate emulsion
(a) Before reaction; (b) After reaction for 1 h; (c) After reaction for 2 h
On the other hand, the hydrolysis and polycondensation take place during the whole process with the alkoxy groups presented, which makes the growing particle form interior uneven crosslinking configuration. Simultaneity, due to the use of water-soluble initiator, the procreant free radicals are more hydrophilic and tend to congregate on the surface of the particles. The crosslinking configuration hinders the free radicals from diffusing to the particle interior. Both of these make the polymerization reaction mainly carry through on the particle surface and obey the surface polymerization mechanism. So the abnormal configuration in the TEM images can be expressed by the cooperational function with the two factors. Additional, the new particles are created constantly during the whole process, the little hypo-particles are captured by the growing particle before they grow, which makes the initial even particle become uneven. The unwonted uneven latex particles with concave-convex on the surface endow the latex with excellent adhesion.
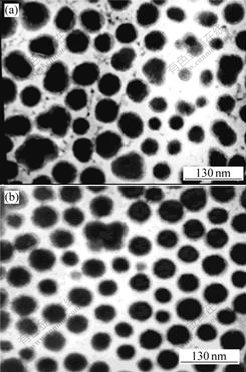
Fig.6 TEM images of C-1706 feeding before reaction and after reaction for 2 h
(a) Before reaction; (b) After reaction for 2 h
3.7 SEM analysis of film configuration
The SEM image of the C-1706 modified acrylate latex film is shown in Fig.7, where the C-1706 content is 6%. Two different phases are apart from each other obviously from the SEM micrograph. The vinyl alkoxy siloxane has characteristic of low surface free energy, softening under low temperature and inconsistent with majority of other polymers. So the C-1706 tends to concentrate on the surface of the film. This result shows that the incorporation of a little amount C-1706 into the backbone has obvious influence on the latex properties.
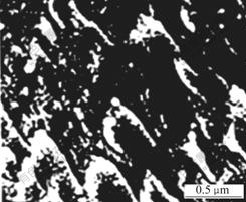
Fig.7 SEM micrograph of coating film of latex
3.8 Ultraviolet aging analysis of film properties
In order to investigate the weather durability and stain resistance of C-1706 modified coating film, the results of ultraviolet aging experiment are shown in Table 5.
Table 5 Aging tests of coating film against UV
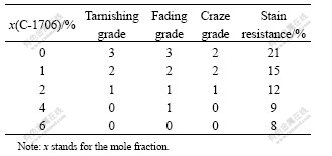
After a period of UV irradiation, some degree tarnishing, fading and craze on the film surface are present, but the wreck degree decreases and the stain resistance improves with the increasing C-1706 content in the emulsion formulation. The main reasons as follows.
Firstly, the vinyl alkoxy siloxane main chain is longer, when the vinyl alkoxy siloxane is grafted to the acrylate polymer backbone, neither can it be enveloped by other branch chain, nor be embedded by acrylate molecule. The resulted modified polymer chain takes on pectination structure. After curing, one end of the C-1706 joins with acrylate molecule, the Si—O chain extends onto the film surface and forms organic siloxane molecule layer. All these lead to the change of chemical composition and structure of the film surface, thereby the film properties are improved.
Secondly, the branch chain of vinyl alkoxy siloxane is alkoxy that decreases the film surface tension and therefore enhances the film stain resistance. The lower surface tension is not only helpful for the wetting and flowing properties for pigments and fillers,but also for the adhesion and penetrating properties to the substrate. Furthermore, the stain resistance of the modified film, one of the most important properties of acrylosiloxane latex, is improved.
And finally, there are some reactive groups in the vinyl alkoxy siloxane. During the film-forming process, the relative groups will react with water, subsequently bonding to the filler or substrate, or self-condensing to form siloxane crosslinks. So the adhesion and weather durability are improved.
4 Conclusions
1) SDS and OP-10 used as multiple emulsifiers can produce coordinated efficiency for the seeded emulsion polymerization of the vinyl triisopropoxy silane modified acrylate latex. The optimal emulsifier content is within 2.4%-3.2%, and the mass ratio of SDS to OP-10 is 1?1-1?2.
2) The vinyl triisopropoxy silane C-1706 as a functional monomer introducing into the all-acrylate latex can improve the weather durability and stain resistance. These benefits of the monomer are derived from the ability to restrain the hydrolysis of the hydrolyzable groups and improve the cross-linking intensity.
3) The results of particle size analysis show that the seeded emulsion polymerization can be used to introduce siloxane bonding in acrylate polymer macromolecule interior and the siloxane modified acrylate copolymer latex possesses narrow distribution of particle size with mean diameter of 51.8-76.6 nm. The excellent properties in weather durability and stain resistance are obtained.
References
[1] PLUEDDEMAN E P. Silane Coupling Agents[M]. 2nd ed. New York: Plenum Press, 1991.
[2] WITUCKI G L. Asilane primer: Chemistry and applications of alkoxysilane[J]. Journal of Coatings Technology, 1993, 65(822): 57-60.
[3] TERTYKH L I. Emulsions of polyethylhydridesiloxane in water for electrophoretic coatings[J]. Colloids and Surfaces, 1999, 152(1): 67-71.
[4] CHEN M J, OSTERHOLTZ F D, PHOL E R. Silanes in high-solids and waterborne coatings[J]. Journal of Coatings Technology, 1997, 69(870): 43-51.
[5] ZHANG Xin-ya, HUANG Hong, CHEN Huan-qin, et al. kinetics and nucleation mechanism of acrylate emulsion polymerization modified by vinyl alkoxy silane[J]. Journal of Chemical Industry and Engineering,2006,57(1): 197-222. (in Chinese )
[6] GUO Ming, SUN Jian-zhong, ZHOU Qi-yun. Synthesis and characterization of polysiloxane/polyacrylate copolymer latexes[J]. Journal of Chemical Engineering of Chinese University, 2002, 16(2): 180-184. (in Chinese)
[7] CHEN M J, CHAVES A, OSTERHOLTZ F D, et al. Silianes in coatings technology[J]. Surface Coating International, 1996, 79(12): 539-545.
[8] LOVELL P A, EL-AASSER M S. Emulsion Polymerization and Emulsion Polymers[M]. New York: Wiley Press, 1997.
[9] POHL E R, OSTERHOLTZ F D. Molecular Characterization of Composite Interfaces[ M ]. New York: Plenum Publishing, 1985.
[10] SIMPSON T R E, PARBHOO B, KEDDIE J L. The dependence of the rate of crosslinking in poly(dimethyl siloxane) on the thickness of coatings[J]. Polymer, 2003, 44(17): 4829-4838.
[11] GRAWE J R, BUFKIN B G. Survey of the applications, properties, and technology of crosslinking emulsions[J]. Journal of Coatings Technology, 1978, 50(643): 67-83.
[12] BOURNE T R, BUFKIN B G, GRAWE J R, et al. Feasibility of using alkoxysilane-functional monomers for the development of crosslinkable emulsions[J]. Journal of Coatings Technology, 1982, 54(684): 69-82.
[13] LARPENT C, TADROS T F. Preparation of microlatex dispersions using oil-in-water microemulsions[J]. Colloid Polymer Science, 1991, 269(11): 1171-1178.
[14] HELDMANN C, CABRERA R I. Influence of nonionic emulsifiers on the properties of vinyl acetate/VeoVa10 and vinyl acetate/ethylene emulsions and paints[J]. Progress in Organic Coatings, 1999, 35(1): 69-77.
Foundation item: Project(2003B10506) supported by Science and Technology Department of Guangdong Province, China
Received date: 2006-10-20; Accepted date: 2006-12-23
Corresponding author: ZHANG Xin-ya, PhD, Associate professor; Tel: +86-20-87112047; E-mail: cexyzh@scut.edu.cn
(Edited by ZHAO Jun)