
Piezoelectric and dielectric properties of Lix(K0.46Na0.54)1-xNb0.86Ta0.1Sb0.04O3 lead-free ceramics
CHEN Zhi-wu(陈志武) 1, HU Jian-qiang(胡建强)2
1. College of Materials Science and Engineering, South China University of Technology, Guangzhou 510640, China;
2. School of Chemical Science, South China University of Technology, Guangzhou 510640, China
Received 22 August 2007; accepted 10 December 2007
Abstract: Lead-free piezoelectric ceramics Lix(K0.46Na0.54)1-xNb0.86Ta0.1Sb0.04O3 (with x ranging from 0 to 0.1) were synthesized by conventional solid state sintering method. The effect of cationic substitution of Li for K and Na in the A sites of perovskite lattice on the structure, phase transition behavior and electrical properties were investigated. Morphotropic phase boundaries(MPB) between orthorhombic and tetragonal phase are found in the composition range of 0.06≤x≤0.08. Analogous to Pb(Zr,Ti)O3, the dielectric and piezoelectric properties are enhanced for the composition near the morphotropic phase boundary. The Li0.06(K0.46Na0.54)0.94- Nb0.86Ta0.1Sb0.04O3 ceramics show excellent electrical properties, that is, piezoelectric constant d33=215 pC/N, planar electromechanical coupling factor kp=41%, dielectric constant  =1 303, and dielectric loss tan δ=2.45%. The results indicate that Lix(K0.46Na0.54)1-x Nb0.86 Ta0.1Sb0.04O3 ceramic is a promising lead-free piezoelectric material.
=1 303, and dielectric loss tan δ=2.45%. The results indicate that Lix(K0.46Na0.54)1-x Nb0.86 Ta0.1Sb0.04O3 ceramic is a promising lead-free piezoelectric material.
Key words: lead-free ceramics; piezoelectric ceramics; piezoelectric property; dielectric property
1 Introduction
The most widely used piezoelectric ceramics are lead oxide based ferroelectrics, especially Pb(Zr1-xTix)O3(PZT). Specifically, compositions are formulated at around x=0.47, corresponding to a morphotropic phase boundary(MPB) separating ferroelectric rhombohedral and tetragonal phases, whereupon the dielectric and piezoelectric properties are greatly enhanced[1]. However, the toxicity of lead oxide and its high vapor pressure during processing have led to a demand for alternative lead-free piezoelectric materials. The search for alternative piezoelectric materials is now focused on alkali niobates, modified bismuth titanates, and systems in which a MPB occurs[2-5]. Among them, (Na0.5K0.5)NbO3 (NKN) has been considered a good candidate for lead-free piezoelectric ceramics because of its strong piezoelectricity and ferroelectricity. The hot pressed NKN ceramics (about 99% of the theoretical density) have been reported to possess a high Curie temperatures (≈420 ℃), a large piezoelectric responses (d33≈160 pC/N), and a high planar coupling coefficient (kp≈45%)[6-9]. However, NKN ceramics sintered by ordinary sintering show relatively lower electrical properties (d33=70 pC/N, kp=25%) due to the difficulty in obtaining a high density by conventional preparation and sintering in air[10]. Therefore, various techniques, such as hot pressing[8], cold-isostatic pressing[11], and spark plasma sintering[12-13], have been utilized to improve the electrical properties of NKN ceramics. Since these techniques were found to be unsuitable for use in industrial production, many studies were conducted by several researchers in order to prepare KNN based ceramics by conventional solid state sintering method and without cold-isostatic pressing(CIP) process. And the result showed that NKN based ceramics doped with Li, Ta, and Sb showed excellent piezoelectric and electromechanical properties due to the formation of a MPB between orthorhombic and tetragonal ferroelectric phases[14-15].
The textured (K0.44Na0.52Li0.04)(Nb0.86Ta0.1Sb0.04)O3 ceramics reported by SAITO et al[16] show excellent properties (d33=416 pC/N, kp=0.61), and proper Ta substitution for Nb could enhance the properties of (K0.44Na0.52Li0.04)(Nb0.96-xTaxSb0.04)O3[17]. For (K0.44Na0.52- Li0.04)(Nb0.86Ta0.1Sb0.04)O3 perovskite ceramics, Li, Na and K occupy the A sites, while Ta, Sb and Nb occupy the B site[16-17] of ABO3 perovskite structure. However, there have been few studies on the effects of Li-substitution (A site substitution) on the properties of this system.
In this work, Lix(K0.46Na0.54)1-xNb0.86Ta0.1Sb0.04O3 piezoelectric ceramics were synthesized by traditional solid state sintering process, without sintering aids, cold-isostatic pressing or special powder handling. The effects of cationic substitution of Li for K and Na in the A sites of perovskite lattice on the structure, phase transition behavior and electrical properties were investigated, to determine the existence of MPB and the enhancement of the properties for Lix(K0.46Na0.54)1-x- Nb0.86Ta0.1Sb0.04O3 systems.
2 Experimental
Lix(K0.46Na0.54)1-xNb0.86Ta0.1Sb0.04O3 piezoelectric ceramics (abbreviated as NKNTS-xLi, with x ranging from 0 to 0.1) were prepared by a conventional mixed-oxide technique using commercially available metal oxides or carbonate powders: Na2CO3, KCO3, LiCO3, Nb2O5, Ta2O5 and Sb2O3. The powders were weighed and mixed well in alcohol with zirconium balls by ball-milling for 10 h. The calcination was then conducted at 850 ℃ for 4 h. The calcined mixture was ball-milled in alcohol again for 6 h. After drying, it was mixed thoroughly with a PVA binder solution and uniaxially pressed into disk samples with a diameter of 20 mm and a thickness of 2 mm. The disk sample was then sintered at 1 100 ℃ for 2 h in air. The crystallite structure of the sintered sample was examined using an X-ray diffractometer (XRD, D/Max-3C, Japan) with Cu Kα radiation. The lattice parameters were refined by the least-square method. Density was measured by the Archimedes method with distilled water.
Silver paste was applied to the top and bottom surfaces of the samples as electrodes. The ceramic samples were polarized under a dc field of 3 kV/mm at 100 ℃ in a silicon oil bath for 15 min. Piezoelectric constant d33 of the samples was measured by means of quasistatic d33 meter (ZJ-3A) based on Berlincourt method. Dielectric constant  was obtained by measuring the capacitance at 1 kHz using an impedance analyzer (HP4192A). Resonance measurements were performed using an impedance analyzer (HP 4294A). The electromechanical coupling factors kp were calculated from the resonance and the anti-resonance frequencies according to ONOE’S formula[18].
was obtained by measuring the capacitance at 1 kHz using an impedance analyzer (HP4192A). Resonance measurements were performed using an impedance analyzer (HP 4294A). The electromechanical coupling factors kp were calculated from the resonance and the anti-resonance frequencies according to ONOE’S formula[18].
3 Results and discussion
Fig.1 shows the dependence of density (ρ) on the chemical composition for the NKNTS-xLi ceramics sintered at 1 100 ℃. The density (ρ) of the ceramics bodies increases with increasing x initially, reaches the maximum at x=0.06(ρ=4.48 g/cm3) and then decreases with further increase in x value. By adding Li2CO3 with the low melting point(618 ℃), the liquid formation promotes the sintering of NKNTS-xLi ceramics, hence leading to an increase in the density of ceramics. Further increasing the Li content makes 1 100 ℃ too high for sintering NKNTS-xLi ceramics and causes the density to decrease.
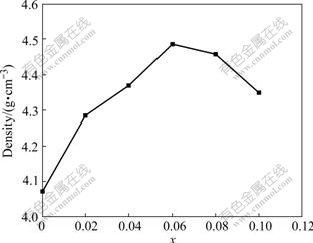
Fig.1 Density as function of composition for NKNTS-xLi system
Fig.2(a) shows the X-ray diffraction(XRD) patterns of NKNTS-xLi ceramics. All the samples are of single perovskite structure and no second phase can be detected, which indicates that Li elements have diffused into the perovskite lattice to form a solid solution. In this case, Li can replace Na and K in the A sites of ABO3 perovskite structure. Fig.2(b) shows the magnification of Fig.2(a) in the range from 40? to 55?. Fig.3 shows the variations of the lattice parameters as a function of Li content. Combining Fig.2(b) and Fig.3, it can be seen that the NKNTS-xLi ceramics have orthorhombic structures in the case of x≤0.06. With increasing Li content(x=0.08, 0.1), however, the structure changes from orthorhombic to tetragonal. The presence of an orthorhombic to tetragonal MPB is indicated in the solid solution range from 0.06<x<0.08, as evidenced by the changes of the peaks of XRD patterns shown in Fig.2 and values of lattice parameters shown in Fig.3. The structure of solid solutions transforms from orthorhombic to tetragonal probably due to a distortion of octahedra caused by the Li+ ions occupying the A site of NKNTS lattice, which slightly increases the tolerance factor of a ABO3 perovskite structure,  , where rA, rB, and rO are the radii of A, B, and O ions, respectively. This somewhat indicates that the orthorhombic and tetragonal structures in NKNTS-xLi have closer energy states.
, where rA, rB, and rO are the radii of A, B, and O ions, respectively. This somewhat indicates that the orthorhombic and tetragonal structures in NKNTS-xLi have closer energy states.

Fig.2 X-ray diffraction patterns of NKNTS-xLi system

Fig.3 Lattice parameter as function of composition for NKNTS-xLi system
Fig.4 shows the dependence of the planar electromechanical coupling factor kp and piezoelectric constant d33 on the chemical composition for the synthesized NKNTS-xLi ceramics. Coupling factor kp increases with x up to 0.06 and then decreases with further increase in x value. At x=0.06, the sample shows the optimal kp of 41%. Variation of the piezoelectric constant d33 with x is almost similar to that of kp. The optimized x value is also about 0.06, giving maximum d33 value of 215 pC/N. Fig.5 shows the dependence of the dielectric constant  and dissipation factor tanδ on the chemical composition for NKNTS-xLi. Dielectric constant
and dissipation factor tanδ on the chemical composition for NKNTS-xLi. Dielectric constant  of the samples increases with increasing x initially, reaches the maximum at x=0.06 (
of the samples increases with increasing x initially, reaches the maximum at x=0.06 ( =1303) and then shows a slight decrease with further increase in x value. However, dissipation factor tanδ of the samples decreases with increasing x initially, reaches the minimum at x=0.06 (tanδ=2.45%) and then increases with further increase in x. In summary, it can be seen that the optimal electromechanical property occurs in NKNTS-0.06Li composition.
=1303) and then shows a slight decrease with further increase in x value. However, dissipation factor tanδ of the samples decreases with increasing x initially, reaches the minimum at x=0.06 (tanδ=2.45%) and then increases with further increase in x. In summary, it can be seen that the optimal electromechanical property occurs in NKNTS-0.06Li composition.

Fig.4 Piezoelectric constant d33 and planar electromechanical coupling factor kp as function of composition for NKNTS-xLi system
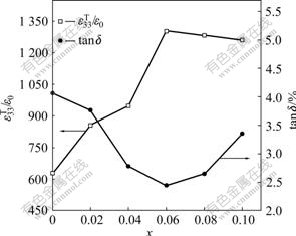
Fig.5 Dielectric constant  and dissipation factor tanδ as function of composition for NKNTS-xLi system
and dissipation factor tanδ as function of composition for NKNTS-xLi system
The content-dependent effect of Li-substitution on the properties of NKNTS-xLi ceramics can be potentially attributed to two factors: chemical modification and microstructure influence of the material. When the amount of Li-substitution is relatively low, chemical modification caused by dissolution of Li+ into the perovskite lattice plays a major role on the properties of NKNTS-xLi ceramics. It is supposed that NKNTS-xLi has ABO3 perovskite structure, and in this case, Li can replace Na and K in A sites of ABO3 perovskite structure. Due to the difference of ionic radius among Li+, K+ and Na+ ions, this substitution will result in an aberrance of crystal structure, which benefits the reorientation of domains during the polarization process. That may be the explanation for the increase of piezoelectric properties. So, analogous to Pb(Zr,Ti)O3, NKNTS-xLi ceramics with good piezoelectric properties can be obtained by partial substitution of A-site ions K+ and Na+ by Li+. When the amounts of Li-substitution are increased enough to cause change in microstructure of the ceramics, the influence of microstructure on the properties of NKNTS-xLi ceramics may become dominant. At the MPB composition (near the NKNTS-0.06Li composition), the crystal structure of ceramics is considered to be a coexistence of orthorhombic and tetragonal phase[1]. As the free energy of orthorhombic phase is close to that of tetragonal phase, when applying an electric field, these two phases are easy to change each other. It is helpful to promoting the movement and polarization of ferroelectric active ion at this case, leading to the increase of dielectric constant  and electromechanical coupling factor kp. The increase in piezoelectric constant d33 at MPB composition may be attributed to an increased flexibility in domain wall[1]. At higher amount of Li-substitution (x>0.06), chemical modification plays a major role on the properties of NKNTS-xLi ceramics again. While at this moment, the decrease in piezoelectric property of the ceramics may be attributed to the high Li-substitution amount[1].
and electromechanical coupling factor kp. The increase in piezoelectric constant d33 at MPB composition may be attributed to an increased flexibility in domain wall[1]. At higher amount of Li-substitution (x>0.06), chemical modification plays a major role on the properties of NKNTS-xLi ceramics again. While at this moment, the decrease in piezoelectric property of the ceramics may be attributed to the high Li-substitution amount[1].
4 Conclusions
1) Lix(K0.46Na0.54)1-xNb0.86Ta0.1Sb0.04O3 lead-free piezoelectric ceramics with x varying from 0 to 0.1 were prepared and their crystalline structure, dielectric and piezoelectric properties were studied. Results of X-ray diffraction(XRD) show that the ceramics exhibit pure perovskite structure. With the increase of Li-substitution, the crystal structure of the samples changes from orthorhombic to tetragonal symmetry.
2) The measurements of electrical properties reveal that Li0.06(K0.46Na0.54)0.94Nb0.86Ta0.1Sb0.04O3 ceramic provides relatively high piezoelectric constant d33 (up to 215 pC/N) and high electromechanical coupling factor kp (up to 41%). The results show that Lix(K0.46Na0.54)1-xNb0.86Ta0.1Sb0.04O3 ceramic is a promising lead-free piezoelectric material.
References
[1] XU Huan-yu. Ferroelectric and piezoelectric materials [M]. Beijing: Science Press, 1978: 125-129. (in Chinese)
[2] CHIANG Y M, FARREY G W, SOUKHOJAK A N. Lead-free high-strain single-crystal piezoelectrics in the alkaline-bismuth- titanate perovskite family [J]. Applied Physics Letters, 1998, 73: 3683-3685.
[3] CHU B J, CHEN D, LI G R, YIN Q R. Electrical properties of Na1/2Bi1/2TiO3-BaTiO3 ceramics [J]. Journal of the European Ceramic Society, 2002, 22: 2115-2121.
[4] GUO Y P, KAKIMOTO K, OHSATO H. Structure and electrical properties of lead-free (Na0.5K0.5)NbO3-BaTiO3 ceramics [J]. Jpnanese Journal of Applied Physics, (Part 1), 2004, 43(9B): 6662-6666.
[5] KAKIMOTO K, MASUDA I, OHSATO H. Ferroelectric and piezoelectric properties of KNbO3 ceramics containing small amounts of LaFeO3 [J]. Japanese Journal of Applied Physics(Part 1), 2003, 42: 6102-6105.
[6] SHIRANE G, NEWNHAM R, PEPINSKY R. Dielectric properties and phase transitions of NaNbO3 and (Na, K)NbO3 [J]. Physical Review, 1954, 96: 581-588.
[7] EGERTON L, DILLON D M. Piezoelectric and dielectric properties of ceramics in the system potassium-sodium niobate [J]. Journal of the American Ceramic Society, 1959, 42: 438-442.
[8] JAEGER R E, EGERTON L. Hot pressing of potassium-sodium niobates [J]. Journal of the American Ceramic Society, 1962, 45: 209-213.
[9] HAERTLING G H. Properties of hot-pressed ferroelectric alkaliniobate ceramics [J]. Journal of the American Ceramic Society, 1967, 50: 329-330.
[10] MAEDER M D, DAMJANOVIC D, SETTER N. Lead free piezoelectric materials [J]. Journal of the Electroceramics, 2004, 13: 385-392.
[11] GUO Y P, KAKIMOTO K, OHSATO H. Phase transitional behavior and piezoelectric properties of Na0.5K0.5NbO3-LiNbO3 ceramics [J]. Applied Physics Letters, 2004, 85: 4121-4123.
[12] ZHANG B P, LI J F, WANG K, ZHANG H L. Compositional dependence of piezoelectric properties in NaxK1-xNbO3 lead-free ceramics prepared by spark plasma sintering [J]. Journal of the American Ceramic Society, 2006, 89: 1605-1609.
[13] LI J F, WANG K, ZHANG B P, ZHANG L M. Ferroelectric and piezoelectric properties of fine-grained Na0.5K0.5NbO3 lead-free piezoelectric ceramics prepared by spark plasma sintering [J]. Journal of the American Ceramic Society, 2006, 89: 706-709
[14] HOLLENSTEIN E, DAVIS M, DAMJANOVIC D, SETTER N. Piezoelectric properties of Li- and Ta-modified (K0.5Na0.5)NbO3 ceramics [J]. Applied Physics Letters, 2005, 87: 182905-182907.
[15] ZANG G Z, WANG J F, CHEN H C, SU W B, WANG C M, QI P, MING B Q, DU J, ZHENG L M, ZHANG S J, SHROUT T R. Perovskite (Na0.5K0.5)1-x(LiSb)xNb1-xO3 lead-free piezoceramics [J]. Applied Physics Letters, 2006, 88: 212908-212910.
[16] SAITO Y, TAKAO H, TANI T, NONOYAMA T, TAKATORI K, HOMMA T, NAGAYA T, NAKAMURA M. Lead-free piezoceramics [J]. Nature(London), 2004, 432: 84-87.
[17] YANG Z P, CHANG Y F, WEI L L. Phase transitional behavior and electrical properties of lead-free (K0.44Na0.52Li0.04)(Nb0.96-xTaxSb0.04)- O3 piezoelectric ceramics [J]. Applied Physics Letters, 2007, 90: 042911-042913.
[18] ONOE M, JUMONJI H. Useful formulas for piezoelectric ceramics resonators and their application to measurement of parameters [J]. Journal of the Acoustical Society of America, 1967, 41: 974-980.
Foundation item: Project(50702022) supported by the National Natural Science Foundation of China; Project(SYSJJ2007-11) supported by the Key Laboratory of Silicate Materials Science and Engineering (Wuhan University of Technology), Ministry of Education, China; Project(KF0701) supported by Low Dimensional Materials and Application Technology (Xiangtan University), Ministry of Education, China
Corresponding author: CHEN Zhi-wu; Tel: +86-20-87111003; E-mail: chenzw@scut.edu.cn
(Edited by YANG Bing)