
Microstructure and property of zinc phosphate coating on
die-casting magnesium alloy AZ91D
LI Guang-yu(李光玉), LIAN Jian-she(连建设), NIU Li-yuan(牛丽媛), JIANG Zhong-hao(江中浩)
Key Laboratory of Automobile Materials, Ministry of Education,
College of Materials Science and Engineering, Jilin University, Changchun 130025, China
Received 20 April 2006; accepted 30 June 2006
Abstract: A surface treatment method was described, which can form a uniform and dense phosphate conversion coating on the die -casting magnesium alloy AZ91D in a non-chromate and non-nitrite bath. The coating consists of Zn3(PO4)2?4H2O, Zn, AlPO4 and MgZn2(PO4)2 analyzed by XRD. The SEM results show that the microstructure of the zinc phosphate coating transfers from flower-like to slab-like crystals with the increase of immersion time of magnesium alloy samples in the phosphating bath. The zinc phosphate coating formed in the bath with immersion time of 1 min is denser because metallic Zn and insoluble phosphate crystals co-deposit on the magnesium alloy surface and the growth of the crystals are restricted by each others. The zinc phosphate coating on the magnesium alloy is used as the base layer for further cataphoric and powder paintings. The cataphoric painting on AZ91D alloy based on phosphate coating has similar adhesion and corrosion-resistance to that based on the chromate conversion coating. But for powder painting, the former exhibits better adhesion property than the latter, due to the uneven microstructure and the enough thickness of the phosphate coating.
Key words: magnesium alloy; AZ91D; phosphate coating; adhesion; anticorrosive
1 Introduction
Surface treatment to prevent corrosion of magn- esium alloys has attracted great interest and relevant studies have been developed quickly in recent years [1-10]. Paint systems are satisfactory on magnesium alloy surface and have been widely used in industries. Proper surface chemical conversion film prior to painting is needed to ensure good contact between paint and metal surface. Several studies of chromate conversion coating, stannate conversion coating, cerium conversion coating and phosphate-permanganate coating were focused on magnesium alloys recently [11-16]. The common dem- erit of these conversions coating is that their thickness is thin (only 0.2-1 μm), which could not satisfy the industrial need. Phosphate coating [17-20] is the most promising substitute to chromate coating that can create good bonding between paint film and metal. Based on this consideration, zinc phosphating process has been widely used in automobile industry as a pretreatment method on steel and aluminum alloy surface. There are only a few reports about phosphating process and phosphating mechanism of magnesium alloy because the phosphatization is rather difficult due to the high chemical activity of magnesium alloy. KOUISNI et al. [21,22] studied the formation and growth of zinc phosphate coating containing Zn3(PO4)2?4H2O on an AM60 magnesium alloy. The influence of zinc ions (Zn2+) and nitrate on the phosphating process and mechanism were investigated. HAN et al. obtained a phosphate coating of Mn3(PO4)2 on AZ31D magnesium alloy in a bath containing phosphate and manganese [23].
In the present study, a zinc phosphating bath was employed to obtain high quality zinc phosphate coatings on the AZ91D magnesium alloy. The microstructures and compositions of the phosphate coatings were investigated by means of SEM and XRD. The quality of coatings including coating’s thickness and corrosion resistance was estimated. In previous study [21, 22, 24], nitrate and nitrite were used as the accelerator agents added in the phosphating bath. However, because nitrite is a carcinogen, NaClO3 was used as an accelerator agent of phosphatization in the present study.
2 Experimental
The substrate material used in this study was die -casting AZ91D magnesium alloy with the dimensions of 50 mm×50 mm×3 mm. The chemical compositions of the alloy are listed in Table 1.
Table 1 Compositions of AZ91D magnesium alloy (mass fraction, %)

The samples were degreased in 10% KOH and rinsed in de-ionized water to remove all the alkali before the zinc phosphating treatment. The ingredients of the zinc phosphating bath are listed in Table 2. The cleaned specimens were then treated in the phosphating bath and were dried. The phosphating temperature was 40-45 ℃.
Table 2 Compositions of phosphating bath
The coating surface was observed by using SEM (JSM-5310, Japan Electronics). The compositions of phosphate coatings were analyzed by using XRD (D/max-2500PC, Cu Ka). Phosphate coating mass was measured according to the method of the report [24].
The salt spray tests were conducted in SF850 salt spray cabinet (Atlas Electric Devices Company). ASTM B117 Standard [25] was adopted in the tests.
For painted samples, the adhesion tests of the painted samples were conducted according to ISO2409 [26], which is a test method for assessing the resistance of paint coatings to separate from substrates when a right-angle lattice pattern is cut onto the coating, pen- etrating to the substrate. The method was carried out as a six-step classification test. The classification of “0” indicates the best adhesion result of the coating and that of “5” indicates the worst adhesion result of the coating.
3 Results and discussion
During phosphating, the reactions on magnesium
alloy surfaces take place on adjacent local polarization sites correspondingly. KOUISNI et al reported [21] that hydrogen evolvement occurred at the phase b (Mg17Al12) of magnesium alloys firstly, as b phase in the magnesium alloys is regarded as the micro cathode primitively.
Hydrogen is given out from micro cathode sites:
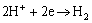 (1)
(1)
The reduction of Eqn.(1) results in the increase of local pH at metal-solution interface, which facilitates the precipitation of insoluble phosphate. Therefore, the reactions of forming insoluble phosphate coating occur:
2Zn2++Zn(H2PO4)2?2H2O+2H2O→Zn3(PO4)2?4H2O+2H2 (2)
2Al3++2H3PO4→2AlPO4+3H2 (3)
Mg+Zn2++Zn(H2PO4)2?2H2O→
MgZn2(PO4)2+4H++2H2O (4)
After the original phosphate film had formed it was regarded as the micro cathode. Reaction (2), (3) and (4) progress continuously, until the nucleation and growth of phosphate crystals to form full covered phosphate film on the substrate surface.
The following reactions occur at micro anode sites:
Mg→Mg2++2e (5)
Al→Al3++3e (6)
During the phosphating process on the micro anodic areas, Zn2+ ions also reduce to zinc and deposit on the surface:
Mg+Zn2+→Zn+Mg2+ (7)
NaClO3 in the phosphating bath is used for accelerating and modifying the coating formation. ClO3 consumes hydrogen ions produce in the phosphating reaction and accelerate the phosphating reaction:
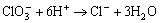 (8)
(8)
Fig.1 shows the SEM image of the zinc phosphate coating on the AZ91D magnesium alloy obtained from the phosphating bath at different immersion time. Fig.1 (a) shows the surface morphology of the phosphated AZ91D sample immersed in the bath for about 0.5 min. Some “white-flower-like” crystals about 2-10 mm form on the substrate but they do not cover the whole surface in 0.5 min. When samples are immersed for 1 min, “white flowers” grow to 5-30 mm (Fig.1 (b)). It is worth to notice that there are some petals of the “white flowers” begin to grow to the “slab-like” crystals. Hence 1 min is a critical time that the phosphate crystals change from flowers to slabs. EDS analysis on these regions
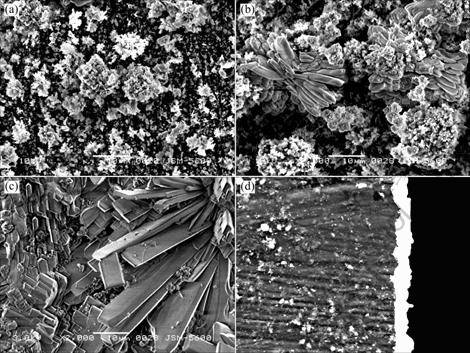
Fig.1 SEM images of zinc phosphate coating on AZ91D magnesium alloy obtained from phosphating bath for different time: (a) 0.5 min; (b) 1 min; (c) 3 min; (d) Cross-sectional morphology of (b)
indicates that these white flowers and slabs consist of mainly Zn3(PO4)2?4H2O and other dark nucleus in Fig.1 (a) and Fig.1 (b) show the mixture of Zn3(PO4)2?4H2O, Zn, AlPO4 and MgZn2(PO4)2. It is seen that there are a lot of clusters of slab-like phosphate crystals after phosphatization for 3 min (Fig.1(c)) and the surface is fully covered by slab-like phosphate crystals.
Fig.2 shows the XRD patterns of the specimens with immersion time 0.5, 1 and 3 min. It is seen that the phases of phosphate coating are Zn3(PO4)2?4H2O, AlPO4 and Zn crystals, which is in agreement with the above discussion on is the formation mechanism of phosphate film. The content of MgZn2(PO4)2 is too slighter and
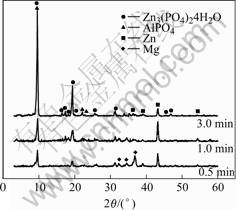
Fig.2 XRD patterns of zinc phosphate coating on AZ91D magnesium alloy formed in phosphating bath for different time
could not be shown in XRD patterns. Magnesium in XRD can still be observed when the phosphated magn- esium samples are dipped for 0.5 min because the film is too thin.
In the XRD pattern of the sample with phosphat- ization time of 1min, both the intensities of (020) plane of Zn3(PO4)2?4H2O of and (101) plane of zinc are higher than that of 0.5 min, which means that both kinds of crystals continue to grow on the surface of magnesium alloy. The crystals of phosphate and metallic zinc co- deposit and their growth were restricted by each other (see Fig.1(b)).
However, in 3 min, the intensity of (020) plane of Zn3(PO4)2?4H2O increases greatly. This implies that the Zn3(PO4)2?4H2O depositions grow quickly and other phases (for example, metallic zinc) stop to deposit.
Fig.3 shows the variations of coating mass (or the coating thickness, if the phosphate coating consists of mainly Zn3(PO4)2?4H2O, 1 g/m2 is equal to about 1 mm) during phosphatization. Zinc phosphate film is thin in the initial 0.5 min. Afterward, because a majority of the substrate was covered by phosphate film, the thickness of coating weights increases to about 3.5 g/m2. It is known that phosphate coating with 2-4.5g/m2 in mass can provide good base prior to paint. In the following discussions about the structure and corrosion property, the phosphate coatings were obtained from the bath with the optimum concentration shown in Table 2 and different phosphating time.
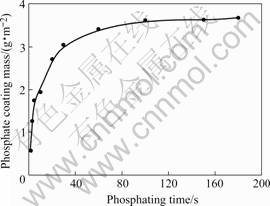
Fig.3 Mass of phosphate coating and immersion time in phosphating bath
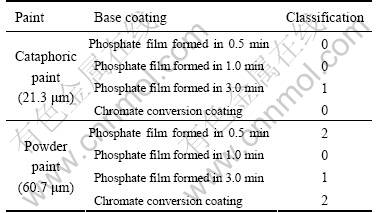
The results of the adhesion test of the paint coatings on the phosphating base and on chromate conversion coating are shown in Table 3, where “0” classification represents the best adhesion and the increase of number means the decrease of adhesion quality according to the ISO2409 standard. Therefore, the traditional chromate conversion coating and the phosphate coating obtained from the bath in 0.5 min are only suitable to the cataphoric paint. The phosphate coating obtained from the bath with immersion time of 1 min is suitable to be the base layer for both the cataphoric paint and powder paint. The adhesion classification of phosphate film formed in the bath in 3 min is “1” and is also suitable for both paints but its adhesion property is not as well as that of formed in the bath in 1 min because its microstructure becomes coarser.
Table 3 Adhesion characteristic results, comparison between cataphoric paint coatings and power paint coatings on phosphate film and chromate conversion film.
For the painted samples, before salt spray tests a beeline was scored on the surface of samples using the knife (ISO2409 Standard) [26]. The nick must be scored to the substrate. The anticorrosion performance is estim- ated by the breadth of the enlarged rusty along one side of the nick after the salt spray test. The results of the salt spray tests of painted AZ91D magnesium alloy based on zinc phosphate coatings formed for 1 min and on chromate conversion coating are shown in Table 4.
Table 4 Breadth of enlarged rusty in lt spray test of painted AZ91D magnesium alloy(mm)
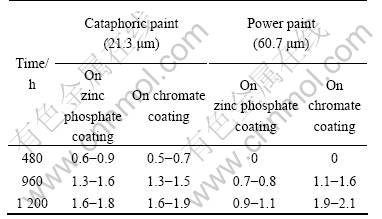
It is seen that the anticorrosion performance of the zinc phosphate coatings formed in 1min on AZ91D magnesium alloy is evidently better than that of the chr- omate coating.
The adhesion and anticorrosion characteristics of the paint + the zinc phosphate coatings formed from the phosphating bath in 1min on AZ91D alloy are better.
4 Conclusions
1) The zinc phosphate coating on the AZ91D magnesium alloy was obtained from a zinc phosphating bath.
2) The chromate conversion coating and the zinc phosphate coatings formed from the phosphating bath in 0.5 min on AZ91D alloy are thinner and can only be used as the base coating for cataphoric paint.
3) The zinc phosphate coatings formed on AZ91D alloy is suitable to be the base coating of both cataphoric and power paint. The paint + phosphate coating obtained from the phosphating bath in 1min on the AZ91D alloy has similar corrosion-resistance in salt spray to that of the paint+chromate conversion coating, but exhibits better adhesion property than the latter.
References
[1] LINDSTR?M R, JOHANSSON L G, SVENSSON J E. The influence of NaCl and CO2 on the atmospheric corrosion of magnesium alloy AZ91 [J]. Mater Corros, 2003, 54(8): 587-594.
[2] SKAR J I. Corrosion and corrosion prevention of magnesium alloys [J]. Mater Corros, 1999, 50(1): 2-6.
[3] FUNATANIA K. Emerging technology in surface modification of light metals [J]. Surf Coat Technol, 2000, 133-134: 264-272.
[4] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys—a critical review [J]. J Alloy Compd, 2002, 336(1-2): 88-113.
[5] HUO H W, LI Y, WANG F H. Corrosion of AZ91D magnesium alloy with a chemical conversion coating and electroless nickel layer [J]. Corros Sci, 2004, 46(6): 1467-1477.
[6] HAWKE D, ALBRIGHT D L. A phosphate-permanganate conversion coating for magnesium [J]. Met Finish, 1995, 93(10): 34-38.
[7] DE1AUNOIS F, PETITJEAN J P, LIENARD P, JACOB-DULIERE M. Autocatalytic electroless nickel-boron plating on light alloys [J]. Surf Coat Technol, 2000, 124(2-3): 201-209.
[8] ROSALBINO F, ANGELINI E, DE NEGRI S, SACCONE A, DELFINO S. Effect of erbium addition on the corrosion behaviour of Mg-Al alloys [J]. Intermetallics, 2005, 13: 55-60.
[9] HOLLSTEIN F, WIEDEMANN R, SCHOLZ J. Characteristics of PVD-coatings on AZ31hp magnesium alloys [J]. Surf Coat Technol, 2003, 162(2-3): 261-268.
[10] CHIU L H, CHEN C C, YANG C F. Improvement of corrosion properties in an aluminum-sprayed AZ31 magnesium alloy by a post-hot pressing and anodizing treatment [J]. Surf Coat Technol, 2005, 191(2-3): 181-187.
[11] GONZALEZ-NUNEZ M A, NUNEZ-LOPEZ C A, SKELDON P. A non-chromate conversion coating for magnesium alloys and magnesium-based metal matrix composites [J]. Corros Sci, 1995, 37(11): 1763-1772.
[12] DABALA M, BRUNELLI K, NAPOLITANO E, MAGRINI M. Cerium-based chemical conversion coating on AZ63 magnesium alloy [J]. Surf Coat Technol, 2003, 172: 227-232.
[13] UMEHARA H, TAKAYA M, TERAUCHI S. Chrome-free surface treatments for magnesium alloy [J]. Surf Coat Technol, 2003, l69-170: 666-669.
[14] KWO Z C, TENG S S. Conversion-coating treatment for magnesium alloys by a permanganate-phosphate solution [J]. Mater Chem Phys, 2003, 80(1): 191-200.
[15] RUDD A L, BRESLIN C B, MANSFELD F. The corrosion protection afforded by rare earth conversion coatings applied to magnesium [J]. Corros Sci, 2000, 42(2): 275-288.
[16] EPPENSREINER F W, JENKINS M R. Chromate conversion coatings [J]. Met Finish, 1999, 97(1): 494-506.
[17] DONOFRIO J. Zinc phosphating [J]. Met Finish, 2000, 98(6): 57-73.
[18] LI G Y, NIU L Y, LIAN J S, JIANG Z H. A black phosphate coating for C1008 steel [J]. Surf Coat Technol, 2004, 176(2): 215-221.
[19] BALA H, TREPAK N M, SZYMURA S. Corrosion protection of Nd-Fe-B type permanent magnets by zinc phosphate surface conversion coatings [J]. Intermetallics, 2001, 9: 515-519.
[20] RETALLICK W B, BRADY M P, HURNPHREY D L. A phosphoric acid surface treatment for improved oxidation resistance of gamma titanium aluminides [J]. Intermetallics, 1998, 6: 335-337.
[21] KOUISNI L, AZZI M, ZERTOUBI M, DALARD F, MAXIMOVITCH S. Phosphate coatings on magnesium alloy AM60 Part 1: study of the formation and the growth of zinc phosphate films [J]. Surf Coat Technol, 2004, 185(1): 58-67.
[22] KOUISNI L, AZZI M, DALARD F, MAXIMOVITCH S. Phosphate coatings on magnesium alloy AM60: Part 2: Electrochemical behaviour in borate buffer solution [J]. Surf Coat Technol, 2005, 192(2-3): 239-246.
[23] HAN E H, ZHOU W Q, SHAN D Y, KE W. Corrosion and protection of magnesium alloy AZ31D by a new conversion coating [J]. Mater Sci Forum, 2003, 419(4): 879-883.
[24] NIU L Y, JIANG Z H, LI G Y, CU C D, LIAN J S. A study and app1ication of zinc phosphate coating on AZ91D magnesium alloy [J]. Surf Coat Technol, 2006, 200, 3021-3026.
[25] ASTM B117-03. Standard Practice for Operating Salt Spray (Fog) Apparatus[S].
[26] ISO Standards 2409-1992. Paints and Varnishes-Cross-Cut Test[S].
(Edited by HE Xue-feng)
Foundation item: Project(2004CB619301) supported by the Foundation of National Key Basic Research and Development Program; Project supported by the 985 Project of Jilin University, China
Corresponding author: JIANG Zhong-hao; Tel: +86-431-5095875; E-mail: jiangzh@jlu.edu.cn