
J. Cent. South Univ. (2018) 25: 1582-1589
DOI: https://doi.org/10.1007/s11771-018-3850-4
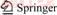
Effect of temperature on floatability and adsorption behavior of fine wolframite with sodium oleate
MENG Qing-you(孟庆有)1, FENG Qi-ming(冯其明)2, OU Le-ming(欧乐明)2
1. School of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China;
2. School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: The influence of pulp temperature on the floatability and adsorption behavior of fine wolframite with sodium oleate was investigated by microflotation experiments, electric conductivity tests, adsorption measurements, and FT-IR analysis. Microflotation results show that fine wolframite with sodium oleate exhibits a good floatability at pH 8–9. Electric conductivity tests indicate that the high temperature enhances the ionization degree and electric mobility of oleate species, then the flotation recovery of fine wolframite and the adsorption amount of sodium oleate are observed to increase with the rise in pulp temperature. The results of adsorption experiments are found to meet Freundlich isotherms successfully, and the isosteric enthalpy (△HΘ) is in conformity with the chemical bonding. The changes in FT-IR analysis provide sufficient evidence that sodium oleate interacts with the metal cations of wolframite surface, and the increase in pulp temperature clearly promotes the chemisorption intensity. These findings will be beneficial to strengthen the flotation behavior of fine wolframite.
Key words: temperature; wolframite; sodium oleate; chemisorption
Cite this article as: MENG Qing-you, FENG Qi-ming, OU Le-ming. Effect of temperature on floatability and adsorption behavior of fine wolframite with sodium oleate [J]. Journal of Central South University, 2018, 25(7): 1582–1589. DOI: https://doi.org/10.1007/s11771-018-3850-4.
1 Introduction
Tungsten plays a dominant role in alloy steels, and its consumption is increasing year by year [1]. Scheelite (CaWO4) and wolframite ((Fe,Mn)WO4) are the main mineral forms of tungsten resource in nature [2]. However, wolframite has always been the primary materials for supplying tungsten products because of its easier beneficiation and lower impurity contents in comparison to scheelite. As for wolframite dressing, high recovery could be achieved with the help of magnetic separation, gravity or a conjunction of them. Over the years, free-dressing wolframite has been mined exceedingly with the continuous consumption of tungsten. The utilization of wolframite slimes, which came into being due to its brittleness in the milling process, has been paid enough attentions. But it is hard to obtain an efficient separation of wolframite in slimes by gravity or magnetic separation. Flotation is a surface-chemistry based process and takes advantage of the difference in the surface property of various minerals, and that it offers the most promising prospects particularly for slimes and finely disseminated particles [3, 4].
However, fine wolframite performs a weak hydrophobicity, and it is very important to select effective methods to enhance its floatability. The pulp temperature has a significant effect on the flotation process. It has been proved that the recovery and grade of floated concentrates were improved for some ores after heating pretreatment [5–7]. In general, the beneficial effect of pulp temperature on the flotation is related to the improvement of collector chemical properties and mineral properties. These changes coming with increasing temperature deeply affect the adsorption process between collector and mineral. FUERSTENAU et al [8] found that the adsorption amounts of oleate and octyl hydroxamate on hematite surfaces increased with the rise in temperature, and the interaction mechanism from physisorption to chemisorption occurred at elevated temperatures. ZHANG [9] also achieved the same conclusion about the adsorption mechanism of sodium oleate on diaspore. The extent of oleate adsorption on the calcite surface increased under the higher temperature at low oleate concentrations, but the adsorption density decreased as pulp temperature increased at higher oleate concentrations [10]. The adsorption of xanthate on chalcopyrite was endothermic and was observed to increase with the increase in temperature [11].
Some researchers have studied the adsorption mechanism of wolframite with collectors. As far as hydroxamate and oleate are concerned, it has been widely accepted that the positive effects for wolframite flotation were mainly connected with the chemisorption which resulted in the form of metal hydroxamate/oleate precipitates on the wolframite surface [12–14]. The temperature influence on the flotation of fine wolframite has not been adequately investigated in the past. Thus this paper aimed to assess the effect of pulp temperature on the floatability and adsorption behavior of sodium oleate on fine wolframite through flotation experiments and adsorption isotherm measurements. The significance of the variation in adsorption intensity with the support of FT-IR was studied and discussed, determining the adsorption mechanism.
2 Materials and methods
2.1 Materials
The purified wolframite used for all experiments was obtained from Yaogangxian mine, Hunan Province, China. The chemical compositions were listed in Table 1, and the purity of wolframite was about 97%. The samples were ground and then elutriated to collect the –10 μm fraction for the microflotation, adsorption and FT-IR tests. Figure 1 shows the particle size distribution of fine wolframite by a laser diffraction particle size analyzer (Mastersize2000, Malvern, England). Hydrochloric acid (HCl) and sodium hydroxide (NaOH) were used as pH regulators. Sodium oleate (C17H33COONa) was used as an anionic collector. All of the reagents utilized in this study were analytical grade. Distilled water was used for all tests.
Table 1 Chemical compositions of purified wolframite sample (mass fraction, %)
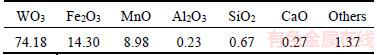

Figure 1 Particle size distribution of fine wolframite
2.2 Experiments
2.2.1 Microflotation experiments
Microflotation experiments were conducted in a mechanical agitation flotation machine. In each test, the mineral suspension was prepared by adding 2.0 g sample to 40 mL distilled water, which kept a desired temperature in the thermostatic water bath. The suspension was adjusted to a desired pH value by adding NaOH or HCl stock solutions and conditioned for 2 min. Then sodium oleate was added to the suspension and conditioned for 30 min. The prepared suspension was then transferred to a 40 mL flotation cell. Flotation froth was scraped out every 10 s for a total of 5 min, and the flotation recovery was calculated according to the mass of sunk and floated products.
The washing experiments of floated wolframite were the same as the above in initial stages. The floated product suspension was centrifuged at 9000 r/min for 10 min, and the solid sample was mixed with 40 mL distilled water at pH 8.0. The suspension was conditioned for 3 min and then was conducted for the flotation. The recovery was the mass percentage of floated products and initial samples.
2.2.2 Electric conductivity tests
The electric conductivity of sodium oleate at different temperature was measured using the conductivity meter (DDS-307, Leici, China).100 mL sodium oleate solution kept for 30 min in the thermostatic water bath, and then the solution was obtained for electric conductivity measurements. The value of electric conductivity at different temperatures was uniformly transformed to that in 25 °C. Three measurements of electric conductivity were conducted, and their average was taken as the final result.
2.2.3 Adsorption measurements
The adsorption amount of sodium oleate on the wolframite surface was determined based on the difference between the initial and final concentrations of the collector solution. 1.0 g sample was conditioned with 40 mL sodium oleate solution at pH 8.0. The obtained suspension was stirred for 4 h in the thermostatic water bath. Experiments showed that the adsorption equilibrium was established within 4 h. The suspension was then centrifuged at 9000 r/min for 10 min, and sodium oleate concentration of the supernatant was measured by the total organic carbon analyzer (TOC-L, Shimadzu, Japan).
2.2.4 FT-IR spectroscopy
The FT-IR spectra of wolframite in the presence of sodium oleate at different temperatures were recorded using the infrared spectrometer (IRAffinity-1, Shimadzu, Japan) in the range 4000-400 cm–1. In the tests, 1 mg desired wolframite sample was mixed with 100 mg spectroscopic grade KBr and pressed into pellets for recording the spectra. The sample treatment process was the same as microflotation test. Subsequently, wolframite samples conditioned for 30 min with sodium oleate were washed for two times using distilled water. The prepared samples for FT-IR analysis were dried in vacuum at 50 °C.
3 Results and discussion
3.1 Microflotation
Figure 2 represents that the flotation behavior of fine wolframite as a function of pH at different temperatures from 25 °C to 60 °C. The results show that fine wolframite displays a relatively good floatability at a pH range from 8.0 to 9.0. The ion-molecular complexes of sodium oleate are viewed as highly active species for the flotation response of wolframite at the optimal pH range, which is benefited from the increase in the effective size of the hydrocarbon chain and low intrinsic solubility [15]. The recoveries of wolframite drop rapidly at pH values less than 8.0 or more than 9.0. On the one hand, the metal cations dissolved from wolframite surfaces in acidic aqueous solution, and there are no active sites on which oleate adsorbs, causing a decrease in the floatability of wolframite. On the other hand, a strong competitive adsorption between oleate ions and hydroxide ions on the wolframite surface exists in strong alkaline solution. OH– ions occupy most of the metal cations of wolframite surface as pH values increase. Metal hydroxyl precipitates are hydrophilic and reduce the wolframite floatability. After the pretreatment with heating from 25 °C to 60 °C, the mass of floated wolframite increases at the entire pH range and rises by 45% at pH 8.0.
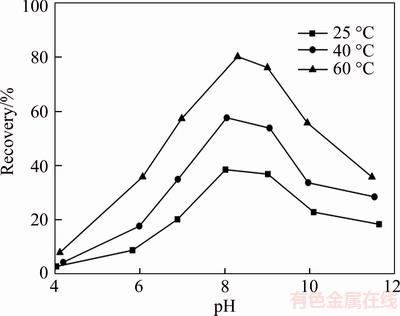
Figure 2 Effect of pH on flotation recovery of fine wolframite (Sodium oleate 1.0×10–4 mol/L)
The results in Figure 3 show that the increase in sodium oleate concentration clearly promotes the flotation recovery of fine wolframite. After the pretreatment with heating, the recovery of fine wolframite increases significantly with the increase in pulp temperatures at low collector initial concentrations and reaches an insignificant increase after sodium oleate concentration more than 4×10–4 mol/L. At the same floatability of fine wolframite, a small amount of heat addition could decrease the dosage of sodium oleate. For the flotation recovery about 55%, the sodium oleate concentration is 2×10–4 mol/L at 25 °C while that is 0.5×10–4 mol/L at 60 °C.
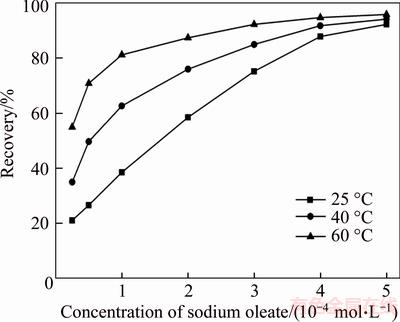
Figure 3 Effect of sodium oleate concentration on flotation recovery of fine wolframite (pH=8.0±0.2)
The relationship between the recovery of floated wolframite and washing time is presented in Figure 4. The results indicate that the recovery of floated wolframite decreases slightly as washing times increase, while a relatively small loss exists at high pulp temperature. The mass of floated wolframite decreases by 52.50%, 46.49%, 27.26% after three washing times at the pulp temperatures of 25, 40, 60 °C, respectively. This finding suggests that the rise in pulp temperature strengthens the adsorption stability of sodium oleate on the wolframite surface.
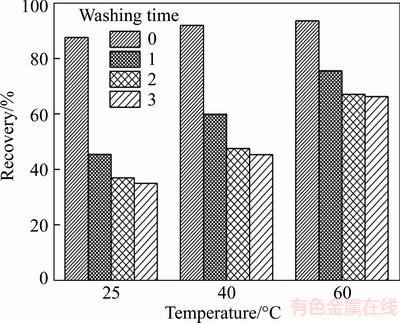
Figure 4 Effect of washing time on recovery of floated wolframite (Sodium oleate 4.0×10–4 mol/L, pH=8.0±0.2)
3.2 Electric conductivity
The electric conductivity of sodium oleate as a function of solution temperature is shown in Figure 5. It is evident that the electric conductivity of sodium oleate increases with the increase in solution temperature. The change trend of electric conductivity is apparent at high concentration of sodium oleate. As well known, the electric conductivity depends on the ion concentration and electric mobility of electrolyte solution, it can be concluded that the increase in temperature is helpful to improve the solution characterization of sodium oleate. The high temperature enhances the electric mobility of oleate species, promoting the diffusion rate of oleate species from the solution to wolframite surface. On the other hand, the ionization degree of sodium oleate increases, which offers greater chances to bond with metal cations of wolframite surface.
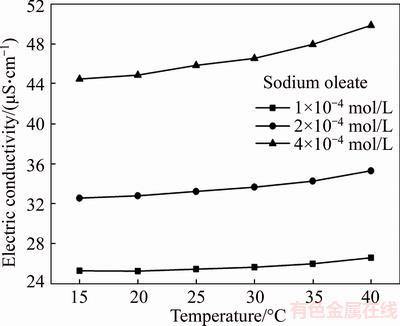
Figure 5 Effect of solution temperature on electric conductivity of sodium oleate
3.3 Adsorption isotherm
Figure 6 presents the adsorption amount of sodium oleate on the wolframite surface as a function of collector initial concentration. It shows a linear increase in the adsorption with the rise in collector concentrations, and that the higher the pulp temperature is, the larger the adsorption amount of sodium oleate will be. Comparing Figure 3 with Figure 6, the flotation responses agree with the adsorption results under the effect of pulp temperature.
A typical adsorption isotherm of sodium oleate on the surface of fine wolframite is shown in Figure 7. The adsorption amount vs the equilibrium collector concentration is found to meet the linear form with R2 values more than 0.95 at temperatures from 25 °C to 60 °C. This adsorption isotherm is referred to the Freundlich isotherm, expressed as Eq.(1).
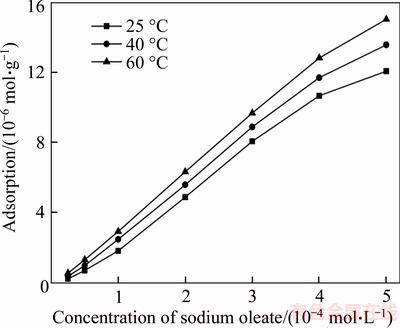
Figure 6 Effect of temperature on sodium oleate adsorption on the wolframite surface (pH=8.0±0.2)
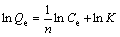 (1)
(1)
where Qe and Ce are the adsorption amount and equilibrium concentration of sodium oleate, respectively. K is the equilibrium constant, and n is related to the strength of the bond formed between the adsorbate and the adsorbent.
The fitting result suggests that the Freundlich adsorption isotherm applies to the adsorption process of sodium oleate, and a multilayer adsorption occurs on the surface of wolframite, in which the outer sodium oleate possibly adsorbs onto the first anchored collector layer through hydrophobic association [12]. Figure 7 shows that the constant n will increase with the increase in temperature, indicating the enhancement in bond strength between sodium oleate and wolframite at the higher temperature. These findings are thus in agreement with the flotation results given in Figure 4.

Figure 7 Freundlich plots for sodium oleate adsorption at different temperatures
The isosteric enthalpy gives an assessment of the heat energy released during the adsorption process, which could be calculated by applying the well known Van’t Hoff equations.
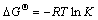 (2)
(2)
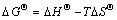 (3)
(3)
Substituting Eq. (2) into Eq. (3) obtains
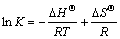 (4)
(4)
where △HΘ is the isosteric enthalpy of adsorption (kJ/mol); R is the molar gas constant (8.314 J/(mol·K)); △GΘ is the Gibbs free energy (kJ/mol); and △SΘ is the isosteric entropy (J/(mol·K)).
△HΘ could be calculated via the slope of the plots of lnK vs 1/T shown in Figure 8. The data fit a single straight line equation with R2 0.990. The △HΘ value thus calculated is –63.21 kJ/mol. According to the research results of Oepen on the relationship between the adsorption enthalpy and interaction forces (Van der Waals force 4–10 kJ/mol, hydrogen bond force 2–40 kJ/mol, Hydrophobic interaction 5 kJ/mol, chemical bond >60 kJ/mol), sodium oleate adsorbs onto the wolframite surface through chemical bonding [16, 17]. YANG [14] investigated that the oleate ions could interact with the ferrous/manganese cations on the surface of wolframite. The value of △HΘ being negative shows that the adsorption of sodium oleate on the wolframite surface is exothermic. In the view of thermodynamics, the rise in temperature may be not conducive to the adsorption of sodium oleate. Actually, the adsorption amount of sodium oleate increases significantly with the increase in pulp temperature. It is well known that the rise of temperature could intensify the activation energy and solubility of oleate species, enhancing the adsorption behavior of sodium oleate on the surface of fine wolframite [18, 19].
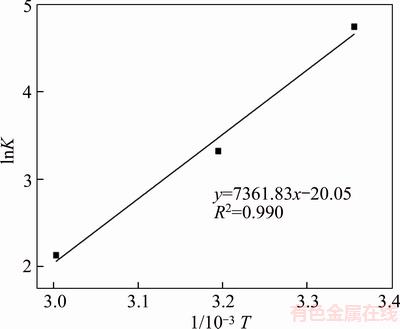
Figure 8 Plots of lnK vs 1/T for sodium oleate adsorption on wolframite surface
3.4 FTIR analysis
The adsorption mechanism of sodium oleate with wolframite was investigated through FT-IR analysis. Figures 9 and 10 present the spectra of sodium oleate, wolframite before and after treated with sodium oleate at different temperatures. In the FT-IR spectrum of sodium oleate (Figure 9), the bands at 2923 cm–1 and 2852 cm–1 are associated with the C—H stretching vibration of the —CH2— and —CH3 groups, respectively. The bands at 1564 cm–1, 1445 cm–1, and 1426 cm–1 are attributed to the —COO— vibration, in which the band at 1564 cm–1 is the —COO— asymmetric stretching vibration, whereas the bands at 1445 cm–1 and 1426 cm–1 are attributed to the —COO— symmetric stretching vibration [20, 21]. In the FT-IR spectrum of wolframite (Figure 10(a)), the characteristic band for wolframite exists at 811 cm–1, corresponding to peaks of [WO6].
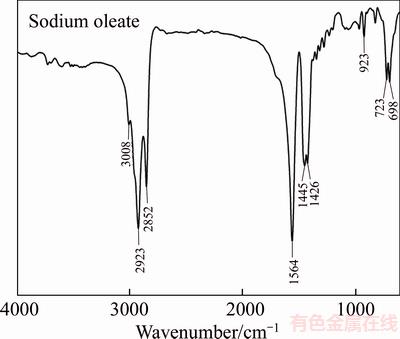
Figure 9 FT-IR spectrum of sodium oleate
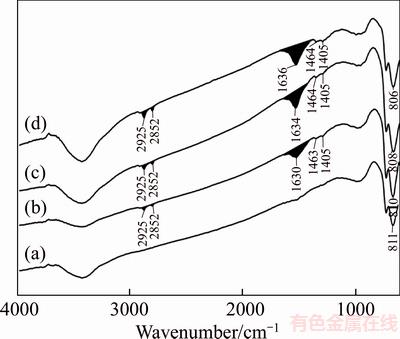
Figure 10 FT-IR spectra of wolframite in absence (a) and presence of sodium oleate 25 °C (b), 40 °C (c) and 60 °C (d)
In the FT-IR spectra of wolframite treated with sodium oleate at different temperatures(Figures 10(b), (c), (d)), The bands around 2925 cm–1 and 2852 cm–1 precisely assign to C—H stretching vibration of sodium oleate. It convincingly demonstrates that sodium oleate adsorbs onto the surface of wolframite. The new bands appeared at around 1630–1640 cm–1,1464 cm–1, and 1405 cm–1 are attributed to the —COO— vibration, and the band frequencies occur obvious changes comparing with that of sodium oleate. The possibility is that oleate ions bond with Fe2+/Mn2+ cations via the oxygen atom of carboxyl groups, resulting in the ferrous/manganese oleate precipitates [22, 23]. The formation of an insoluble layer of ferrous oleate (solubility product Ksp= 10–15.4) and manganese oleate (Ksp=10–15.3) on the wolframite surface increase its hydrophobicity.
As seen from Figure 10, the surface areas at 1630–1640 cm–1, 2925 cm–1 and 2852 cm–1 for wolframite with sodium oleate increase significantly at the temperature range from 25 °C to 60 °C. These results suggest that the rise in pulp temperature could enhance the chemisorption intensity and adsorption density of sodium oleate on the surface of wolframite [24], which agree with the results of adsorption and flotation behavior.
4 Conclusions
1) Pulp temperature has a significant influence on the flotation behavior of wolframite. An increase in temperature considerably improves the floatability of fine wolframite, and a small amount of heat addition could decrease the dosage of sodium oleate.
2) The adsorption measurements indicate that the adsorption amount of sodium oleate increases significantly as the pulp temperature increases. The Freundlich isotherms are identified to the adsorption process of collector, and the isosteric enthalpy of adsorption suggests that sodium oleate adsorbs onto the wolframite surface through the chemical bonding.
3) The FT-IR results confirm that sodium oleate adsorbs chemically onto the surface of wolframite. The chemisorption in the form of ferrous/manganese oleate precipitates is further enhanced with the rise of pulp temperature, giving sufficient evidence for the flotation response.
References
[1] KOUTSOSPYROS A, BRAIDA W, CHRISTODOULATOS C, DERMATAS D, STRIGUL N. A review of tungsten: From environmental obscurity to scrutiny [J]. Journal of Hazardous Materials, 2006, 136(1): 1–19. DOI: 10.1016/ j.jhazmat.2005.11.007.
[2] LASSNER E, SCHUBERT W D. Tungsten properties, chemistry, technology of the element, alloys, and chemical compounds [M]. Boston: Kluwer Academic/Plenum Publishers, 1999.
[3] QIN Wen-qing, REN Liu-yi, XU Yang-bao, WANG Pei-pei, MA Xi-hong. Adsorption mechanism of mixed salicyhydroxamic acid and tributyl phosphate collectors in fine cassiterite electro-flotation system [J]. Journal of Central South University, 2012, 19(6): 1711–1717. DOI: 10.1007/ s11771-012-1197-9.
[4] LUO Xi-mei, YIN Wan-zhong, WANG Yun-fan, SUN Chuan-yao, MA Ying-qiang, LIU Jian. Effect and mechanism of dolomite with different size fractions onhematite flotation using sodium oleate as collector [J]. Journal of Central South University, 2016, 23(3): 529–534. DOI: 10.1007/s11771-016-3099-8.
[5] SU F W, HANUMANTHA R K, FORSSBERG K S E, SAMSKOG P O. The influence of temperature on the kinetics of apatite flotation from magnetite fines [J]. International Journal of Mineral Processing, 1998, 54(3/4): 131–145. DOI: 10.1016/S0301-7516(98)00021-0.
[6] CASTRO S, BORREGO A G. The influence of temperature during flotation of celestite and calcite with sodium oleate and quebracho [J]. International Journal of Mineral Processing, 1996, 46(1): 35–52. DOI: 10.1016/0301- 7516(95)00059-3.
[7] LAZROV D, ALEXANDROVA L, NISHKOV I. Effect of temperature on the kinetics of froth flotation [J]. Minerals Engineering, 1994, 7(4): 503–509. DOI: 10.1016/0892- 6875(94)90163-5.
[8] FUERSTENAU D W, RAGHAVAN S. Some aspects of the thermodynamics of flotation [M]. New York: Gaudian Memorial, Vol. 1. AIME, 1976.
[9] ZHANG Guo-fan, CHEN Qi-yuan, FENG Qi-ming, ZHANG Ping-min. Influence of temperature on adsorption of sodium oleate on surface of diaspore [J]. Chinese Journal of Nonferrous Metals, 2004, 14(6): 1042–1046. (in Chinese)
[10] YOUNG C A, MILLER J D. Effect of temperature on oleate adsorption at a calcite surface: An FT-NIR/IRS study and review [J]. International Journal of Mineral Processing, 2000, 58(1–4): 331–350. DOI: 10.1016/S0301-7516(99)00057-5.
[11] MUSTAFA S, HAMID A, NAEEM A. Temperature effect on xanthate sorption by chalcopyrite [J]. Journal of Colloid and Interface Science, 2004, 275(2): 368–375. DOI: 10.1016/j.jcis.2004.02.007.
[12] HU Yue-hua, WANG Dian-zuo, XU Zheng-he. A study of interactions and flotation of wolframite with octyl hydroxamate [J]. Minerals Engineering, 1997, 10(6): 623–633. DOI: 10.1016/S0892-6875(97)00041-1.
[13] MENG Qing-you, FENG Qi-ming, OU Le-ming. Flotation behavior and adsorption mechanism of fine wolframite with octyl hydroxamic acid [J]. Journal of Central South University, 2016, 23: 1339–1344. DOI: 10.1007/s11771- 016-3185-y.
[14] YANG Si-yuan, FENG Qi-ming, QIU Xian-yang, GAO Yu-de, XIE Zhen-fu. Relationship between flotation and Fe/Mn ratio of wolframite with benzohydroxamic acid and sodium oleate as collectors [J]. Physicochemical Problems of Mineral Processing, 2014, 50(2): 747–758. DOI: 10.5277/ ppmp130226.
[15] PARAPARI P S, IRANNAJAD M, MEHDILO A. Modification of ilmenite surface properties by superficial dissolution method [J]. Minerals Engineering, 2016, 92: 160–167. DOI: 10.1016/j.mineng.2016.03.016.
[16] OEPEN B, KORDEL W, KLEIN W. Sorption of nonpolar and polar compounds to soils: Processes, measurement and experience with the applicability of the modified OECD-guideline 106 [J]. Chemosphere, 1991, 22: 285–304. DOI: 10.1016/0045-6535(91)90318-8.
[17] FENG Qi-ming, XIAO Ya-xiong, LU Yi-ping. Effects of temperature on adsorption amount of sodium oleate on surface of diaspore [J]. The Chinese Journal of Nonferrous Metals, 2015, 25(9): 2582–2587. (in Chinese)
[18] PARIA S, KHILAR K C. A review on experimental studies of surfactant adsorption at the hydrophilic solid–water interface [J]. Advances in Colloid and Interface Science, 2004, 110(3): 75–95. DOI: 10.1016/j.cis.2004.03.001.
[19] LI Xiao, XU Gui-ying, ZHANG Zhi-qing, WANG You-bao, LI Gan-zuo. Adsorption of sodium oleate at the interface of oil sand/aqueous solution [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2003, 224(1–3): 199–206. DOI: 10.1016/S0927-7757(03)00328-5.
[20] LIU Wei-jun, ZHANG Jie, WANG Wei-qing, DENG Jie, CHEN Bing-yan, YAN Wu, XIONG Shu-qing, HUANG Yang, LIU Jing. Flotation behaviors of ilmenite, titanaugite, and forsterite using sodium oleate as the collector [J]. Minerals Engineering, 2015, 72: 1–9. DOI: 10.1016/j.mineng. 2014.12.021.
[21] N JERA J J. Phase transition behavior of sodium oleate aerosol particles [J]. Atmospheric Environment, 2007, 41(5): 1041–1052. DOI: 10.1016/j.atmosenv.2006.09.016.
JERA J J. Phase transition behavior of sodium oleate aerosol particles [J]. Atmospheric Environment, 2007, 41(5): 1041–1052. DOI: 10.1016/j.atmosenv.2006.09.016.
[22] MEHDILO A, IRANNAJAD M. Comparison of microwave irradiation and oxidation roasting as pretreatment methods for modification of ilmenite physicochemical properties [J]. Journal of Industrial and Engineering Chemistry, 2016, 33: 59–72. DOI: 10.1016/j.jiec.2015.09.018.
[23] THISTLETHWAITE P J, HOOK M S. Diffuse reflectance fourier transform infrared study of the adsorption of oleate/oleic acid onto titania [J]. Langmuir, 2000, 16(11): 4993–4998. DOI: 10.1021/la991514i.
[24] NURI O S, MEHDILO A, IRANNAJAD M. Influence of microwave irradiation on ilmenite surface properties [J]. Applied Surface Science, 2014, 311: 27–32. DOI: 10.1016/ j.apsusc.2014.04.187.
(Edited by HE Yun-bin)
中文导读
油酸钠作用下温度对微细粒黑钨矿可浮性和吸附行为的影响
摘要:通过单矿物浮选试验、电导率测试、吸附量测定和红外光谱分析,研究了温度对油酸钠在微细粒黑钨矿表面吸附行为及其可浮性的影响。结果表明,油酸钠作捕收剂,微细粒黑钨矿在pH值8~9区间具有良好的可浮性;矿浆温度升高有助于增强油酸组分的电离度和迁移率,进而增加油酸钠在矿物表面的吸附量和黑钨矿的浮选回收率。吸附试验结果表明油酸钠在黑钨矿表面吸附符合Freundlich等温线,由吸附焓变(ΔHΘ)推断油酸钠在黑钨矿表面为化学吸附。红外光谱分析充分证明了油酸钠能够与黑钨矿表面的金属离子发生反应,且温度越高化学吸附作用越强。这些结果将有助于强化微细粒黑钨矿的浮选行为。
关键词:温度;黑钨矿;油酸钠;化学吸附
Foundation item: Project(51704058) supported by the National Natural Science Foundation of China
Received date: 2017-01-19; Accepted date: 2017-03-28
Corresponding author: FENG Qi-ming, PhD, Professor; Tel: +86–731–88836817; E-mail: feng_309@csu.edu.cn; ORCID: 0000-0002- 7955-0376