NaOH水热转化-H2SO4浸出法从硫酸法钛白酸解残渣(黑泥)中回收利用钛
来源期刊:中国有色金属学报(英文版)2016年第6期
论文作者:孟凡成 薛天艳 刘亚辉 张国之 齐涛
文章页码:1696 - 1705
关键词:黑泥;钛回收;NaOH水热转化;水洗;H2SO4浸出
Key words:tionite; titanium recovery; NaOH hydrothermal conversion; water washing; H2SO4 leaching
摘 要:提出一种从黑泥中回收利用钛的新工艺,该工艺包括NaOH水热转化、水洗和H2SO4浸出制备TiO2。在优化的反应条件下,即NaOH溶液浓度为50%(质量分数)、NaOH/黑泥质量比为4:1、反应温度为240 °C、反应时间为1 h和氧气分压为0.25 MPa,钛转化率可达97.2%,主要含钛产物是Na2TiO3。非目标产物Na2TiSiO5在水洗中保持稳定,在水热反应中提高NaOH浓度可以抑制Na2TiSiO5的生成。水热产物经过水洗后,97.6%的Na+可以回收。含有NaOH的溶液经过浓缩之后可以回用。在较低温度下,水洗物料中96.7%的钛能被较低浓度的硫酸浸出得到钛液。利用所得钛液进一步制备合格TiO2产品。
Abstract: To recover titanium from tionite, a new process consisting of NaOH hydrothermal conversion, water washing, and H2SO4 leaching for TiO2 preparation was developed. The experimental results show that under the optimum hydrothermal conversion conditions, i.e., 50% NaOH (mass fraction) solution, NaOH/tionite mass ratio of 4:1, reaction temperature of 240 °C, reaction time of 1 h and oxygen partial pressure of 0.25 MPa, the titanium was mainly converted into Na2TiO3, and the conversion was 97.2%. The unwanted product Na2TiSiO5 remained stable in water washing, and its formation was prevented by improving NaOH concentration. In water washing process, about 97.6% of Na+ could be recycled by washing the hydrothermal product. The NaOH solutions could be reused after concentration. 96.7% of titanium in the washed product was easily leached in H2SO4 solution at low temperatures, forming titanyl sulfate solution to further prepare TiO2.
Trans. Nonferrous Met. Soc. China 26(2016) 1696-1705
Fan-cheng MENG1,2,3, Tian-yan XUE1,2, Ya-hui LIU1,2, Guo-zhi ZHANG1,2,3, Tao QI1,2
1. National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China;
2. Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China;
3. University of Chinese Academy of Sciences, Beijing 100049, China
Received 10 July 2015; accepted 10 November 2015
Abstract: To recover titanium from tionite, a new process consisting of NaOH hydrothermal conversion, water washing, and H2SO4 leaching for TiO2 preparation was developed. The experimental results show that under the optimum hydrothermal conversion conditions, i.e., 50% NaOH (mass fraction) solution, NaOH/tionite mass ratio of 4:1, reaction temperature of 240 °C, reaction time of 1 h and oxygen partial pressure of 0.25 MPa, the titanium was mainly converted into Na2TiO3, and the conversion was 97.2%. The unwanted product Na2TiSiO5 remained stable in water washing, and its formation was prevented by improving NaOH concentration. In water washing process, about 97.6% of Na+ could be recycled by washing the hydrothermal product. The NaOH solutions could be reused after concentration. 96.7% of titanium in the washed product was easily leached in H2SO4 solution at low temperatures, forming titanyl sulfate solution to further prepare TiO2.
Key words: tionite; titanium recovery; NaOH hydrothermal conversion; water washing; H2SO4 leaching
1 Introduction
TiO2 is an important inorganic chemical reagent that is widely used in white pigment, plastic, paper, and photocatalyst [1-3]. The industrial technologies for TiO2 production mainly include the sulfate process and the chloride process [4]. In China, more than 90% of TiO2 is produced via the sulfate process [5]. One of the major solid wastes in the sulfate process is the undissolved residue generated during the digestion of titanium-based minerals [6,7]. This undissolved residue, known as tionite in some literatures [8,9], is a fine-grained mud composed of titanium, iron, and silicon minerals. Up to 0.4-0.6 tons of tionite (wet basis) are generated per ton of produced titanium oxide, and the content of TiO2 in the dry basis of tionite is 35%-50% (mass fraction). In this sense, tionite can be regarded as a valuable secondary titanium resource. Residual H2SO4 and particular heavy metal ions are also present in fresh tionite [10]. Tionite is listed on the National Hazardous Waste List of China (waste code: 261-056-34). At present, tionite usually neutralizes with lime or limestone and is directly stacked in landfills. More than 10 million tons of tionite are discharged per year in China. The treatments previously mentioned not only create great environmental risks and dilemma in disposal space but also cause a significant loss of titanium resource.
Several processes have been reported for the reuse of tionite or the recovery of titanium from tionite. As an example, tionite has been utilized in construction materials, such as clay bricks [8], sulfur polymer cement [11], and ceramic material [9,12]. However, assuring the inertness of hazardous species in the obtained products is a significant concern, and the titanium resource in tionite is wasted. In addition, physical separation methods combining gravity concentration, flotation, or magnetic separation for recycling titanium minerals from tionite have gained significant attention [13-15]. However, these physical separation methods only recycle part of the ilmenite from tionite, and the rest of titanium minerals, such as rutile and anatase, could not be recovered. Due to its fine particle size and high content of impurities, tionite cannot be used as the raw material in the chloride process for TiO2 production. The high content of silicon and rutile (H2SO4 can hardly digest rutile) has also impeded the reuse of tionite in sulfate process. Thus, it is of great importance to develop new method for recovering titanium from the tionite.
Hydrometallurgical processes offer easy and eco-friendly approaches of recovering valuable metals from solid industrial wastes [16,17]. Hydrothermal conversion in alkali solution is an effective method for the conversion or decomposition of titanium and silicon minerals [18,19]. Na2TiO3, which was originally obtained by roasting titanium-based minerals with molten NaOH, was the ideal titanate for TiO2 pigment production in our previous studies [20, 21]. However, no studies have been reported on the preparation of Na2TiO3 via NaOH hydrothermal method as well as the competitive formation of Na2TiSO5 and the whole process for recovering titanium.
To avoid environmental issues and recover titanium from tionite, this study presented a new eco-friendly process. The flow sheet of this process combining NaOH hydrothermal conversion, water washing, and H2SO4 leaching for TiO2 production is shown in Fig. 1. This new process seems to be more energy-saving and can consume solid waste tionite and even the waste acid produced in the sulfate process. It appears a promising supplement to the existing sulfate process of TiO2 industry. The effects of key factors on the NaOH hydrothermal conversion of tionite and the recovery of titanium from tionite were investigated.
2 Experimental
2.1 Materials
The fresh tionite (wet basis) used in this study was supplied by Shandong Dongjia Group Co., Ltd., China. Prior to the experiments, the fresh tionite was washed with water and filtered for the removal of acid. Next, the tionite was treated with a dilute alkaline solution to neutralize the residual acid. The filtration cake was then dried at 100 °C overnight to obtain dry tionite. The chemical composition of dry tionite is listed in Table 1.

Fig. 1 Flow sheet of new process for recovery of titanium from tionite
The content of TiO2 in dry basis is 46.4%. The X-ray diffraction (XRD) analysis of dry tionite shown in Fig. 2 indicates that rutile, anatase, ilmenite, and quartz are the main phases of tionite. Some titanium and silicon oxides may also exist in tionite as amorphous form [10].
Analytical-grade solid NaOH and deionized water were used throughout the experiments. Commercial- grade pure oxygen was used in hydrothermal conversion.
2.2 Experimental procedures
2.2.1 Hydrothermal conversion
The hydrothermal conversion experiments were carried out in a 1 L nickel autoclave, which was thermostatically controlled within ±2 °C. A mechanical agitator with a stirring speed of 250 r/min was applied to keeping the slurry suspended. For each experiment, the dry tionite was dispersed in the NaOH solution in the autoclave with the working volume of 65%-70%. Different amounts of O2 was injected into the autoclave prior to the reaction. Then, the autoclave was heated in advance for about 1.5 h to reach the selected temperature. Once the temperature was reached, the time was set as the starting point of the reaction time. After the reaction, the mixture in the autoclave was filtered. The filter cake (hydrothermal product) was sampled and dried for further analysis.
Table 1 Composition analysis results of tionite (mass fraction, %)


Fig. 2 XRD pattern of dry tionite
2.2.2 Water washing for NaOH recirculation
Water washing experiments were performed in a flask reactor heated by an oil bath. The filter cakes obtained from hydrothermal conversion were washed with water at 55 °C. CaO was added into the water washed solution to remove the Si impurities. The water washed solution after desilicication and the hydrothermal converted solution obtained after hydrothermal conversion were then concentrated using a vacuum rotary evaporator to recycle the NaOH solution.
2.2.3 Acid leaching for TiO2 production
In the acid leaching experiments, the washed filter cake was mixed with H2SO4 solution in a flask reactor heated by an oil bath. After the reaction, the mixture was filtered to obtain the titanyl sulfate solution. The prepared titanyl sulfate solution was hydrolyzed at boiling temperature, and the hydrolysis product was washed and calcinated to prepare TiO2.
2.3 Characterization
The XRD patterns of the solid samples were obtained using an X-ray diffractometer (X’Pert PRO MPD, PANalytical, Netherlands) with Cu Kα radiation. The chemical compositions of the solid samples and the solutions were determined via inductively coupled plasma optical emission spectrometry (ICP-OES) (Optimal 5300DV, Perkin-Elmer, USA).
Titanium in tionite could be converted into Na2TiO3 or Na2TiSiO5 via NaOH hydrothermal conversion. The solid sample was dissolved in 3.1% HCl (mass fraction) solution. The dissolution process occurred according to the following reactions:
Na2TiO3+4HCl=TiO2++4Cl-+2Na++2H2O (1)
Na2TiSiO5+4HCl=TiO2++4Cl-+2Na++H2SiO3+H2O (2)
After dissolution of the hydrothermal product (the titanium in the unreacted tionite cannot be dissolved), titanium conversion was calculated via the analysis of ICP-OES.
The morphologies of the solid samples were characterized by scanning electron microscopy (SEM) (JSM-6700, JEOL, Japan) equipped with energy dispersive X-ray spectroscopy (EDS) (INCA X-MAX, Oxford Instruments, US).
3 Results and discussion
3.1 Hydrothermal conversion
3.1.1 Effects of NaOH concentration and NaOH/tionite mass ratio
The effects of NaOH concentration and NaOH/tionite mass ratio on the conversion of titanium are shown in Fig. 3. The results indicate that the conversion of titanium increased with increasing the NaOH/tionite mass ratio at a known NaOH concentration. The conversion rapidly increased from 59.2% to 84.1% when the NaOH/tionite mass ratio increased from 2:1 to 6:1 in 40% NaOH solution. The theoretical mass ratio for the complete reaction is 0.77:1. Excess NaOH, which acts as a mineralizer in the hydrothermal process, is necessary to ensure that the reaction goes into completion. The results shown in Fig. 3 also indicate that the conversion of titanium increased with increasing the NaOH concentration. Furthermore, the conversion of titanium slightly increased when the NaOH concentration increased from 50% to 60% at NaOH/ tionite mass ratios above 4:1.

Fig. 3 Effects of NaOH concentration and NaOH/tionite mass ratio on conversion of titanium
The XRD patterns of the hydrothermal products obtained at different NaOH concentrations and NaOH/ tionite mass ratios are shown in Fig. 4. The main phases of the solid samples obtained at 50% NaOH solution were α-Na2TiO3, Na2SiO3, and a small amount of Na2TiSiO5. Na2CO3 was detected due to the reaction of residual NaOH in hydrothermal product with CO2 in drying the hydrothermal sample for analysis. Fewer peaks of iron components in the hydrothermal products were detected because iron oxide may react with NaOH and exist with low crystallinity. α-Na2TiO3 (JCPDS No. 028-1152) was a metastable phase of Na2TiO3 according to the previous study [20,21]. However, with the decrease of NaOH concentration, the amount of Na2TiSiO5 increased. The dominant phase of titanium was Na2TiSiO5 in the hydrothermal products obtained in 30% and 40% NaOH solutions. Some unreacted rutile, anaste, and ilmenite were also observed. No peaks of Na2SiO3 were detected in the above hydrothermal products, which indicates that most of Si and a part of Ti reacted with NaOH to form Na2TiSiO5. Na2TiSiO5 (JCPDS No. 048-1892), namely natisite, is a stable titanosilicate with a dense structure [22].
The hydrothermal products obtained in 50% NaOH solution with NaOH/tionite mass ratio of 4:1 and 40% NaOH solution with NaOH/tionite mass ratio of 4:1 shown in Fig. 4 were named hydrothermal products A and B, respectively. The SEM images and EDS results of hydrothermal products A and B are shown in Fig. 5. The results show that α-Na2TiO3 was somewhat porous and full of loose rough-etched traces. The rough-etched surface and porous structure could endow α-Na2TiO3 good ion-exchange ability when it was treated with water, contributing to the subsequent Na+ recycling. Whereas, with a smooth square sheet structure, Na2TiSiO5 remained stable in water (Section 3.2) and slowly disintegrated in H2SO4 solution to form a large amount of gel that contained silicon and titanium, which would result in significant loss of Na and Ti components.
Thus, the formation of Na2TiSiO5 must be avoided in NaOH hydrothermal conversion. α-Na2TiO3 was the ideal target product in NaOH hydrothermal conversion, and 50% NaOH solution was favorable for the formation of α-Na2TiO3.

Fig. 4 XRD patterns of hydrothermal products obtained at different NaOH concentrations and NaOH/tionite mass ratios

Fig. 5 SEM images of hydrothermal product A (a) and hydrothermal product B (b), and EDS point analysis of spot 1 (c) and spot 2 (d)
3.1.2 Effect of reaction temperature and reaction time
The effects of reaction temperature and reaction time on the titanium conversion are shown in Fig. 6. The results indicate that the conversion of titanium reached 87.7% at 240 °C for 1 h, whereas a less conversion of 68.3% was observed at 210 °C for 1 h. The results indicate that increasing the temperature was favorable to titanium conversion, which could be attributed to the fact that high temperatures can change the chemical equilibrium constants and boost the reaction rates [23]. Furthermore, the reaction time had less influence on the conversion of titanium after 1 h, especially at high temperatures, meaning 1 h was the optimum reaction time.

Fig. 6 Effects of reaction temperature, reaction time, and oxygen partial pressure on conversion of titanium
The XRD patterns of the hydrothermal products obtained at different reaction temperatures and reaction time are shown in Fig. 7. This result indicates that a long reaction time favored the formation of Na2TiSiO5 probably because the produced metastable α-Na2TiO3 transformed into stable Na2TiSiO5. In comparison with product obtained at 240 °C for 4 h, those products obtained at 240 °C for 1 h had less Na2TiSiO5. As shown in Fig. 7, more Na2TiSiO5 was also observed with increasing the reaction temperature. Although less Na2TiSiO5 formed at 210 °C, the conversion of titanium remained lower than 83% even for a long reaction time of 4 h (Fig. 6). As shown in Fig. 7, some unreacted rutile, anaste, and ilmenite still remained in the hydrothermal product at 210 °C. When the temperature increased to 270 °C, more than 94% of titanium conversion of tionite could be obtained, but much Na2TiSiO5 was observed. The formation of Na2TiSiO5 might be attributed to the combination of Na2TiO3 and Na2SiO3 at such a high temperature.

Fig. 7 XRD patterns of hydrothermal products obtained at different reaction temperatures and reaction time
3.1.3 Effect of oxygen partial pressure
Different amounts of O2 were injected into the autoclave prior to the hydrothermal reaction to investigate the effect of oxygen partial pressure. The results shown in Fig. 6 indicate that the conversion of titanium increased obviously with increasing the oxygen partial pressure with the reaction time of 1 h. At an oxygen partial pressure of 0.25 MPa for 1 h, the conversion of titanium was 97.2%, which was much higher than that without adding O2. Further increasing the O2 partial pressure to 0.50 MPa led to no evident increase of the conversion of titanium with a reaction time of 1 h. Thus, 0.25 MPa of oxygen partial pressure was optimal for this reaction. The XRD patterns of hydrothermal products shown in Fig. 8 indicate that the addition of oxygen facilitated the decomposition of ilmenite in tionite, and the dominant phase of titanium in hydrothermal products remained Na2TiO3.
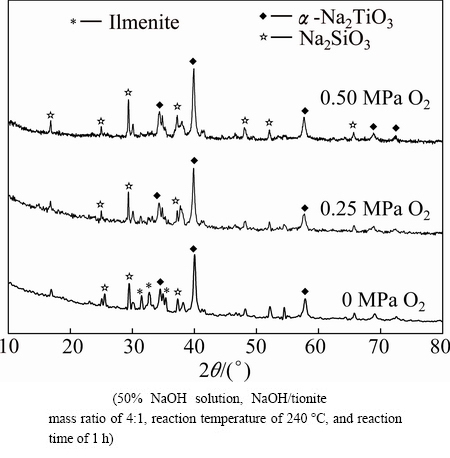
Fig. 8 Effect of oxygen partial pressure on XRD patterns of hydrothermal products
3.1.4 Mechanism on NaOH hydrothermal conversion of tionite
Titanium components undergo a dissolution/ precipitation process in NaOH solution under hydrothermal conditions [24]. The reaction of titanium-based minerals in tionite with NaOH solution resulted in the release of titanium as Ti(IV), probably in the form of 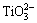 . Among many kinds of meta-silicic ions,
. Among many kinds of meta-silicic ions,  was the predominant ion in highly concentrated NaOH solutions [25]. The dissolved Ti(IV) could form Na2TiO3 or integrate with
was the predominant ion in highly concentrated NaOH solutions [25]. The dissolved Ti(IV) could form Na2TiO3 or integrate with  to form Na2TiSiO5. Thus, the overall hydrothermal reactions could be described as follows:
to form Na2TiSiO5. Thus, the overall hydrothermal reactions could be described as follows:
TiO2+2OH-→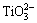 +H2O (3)
+H2O (3)
2Na++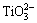 →Na2TiO3(s) (4)
→Na2TiO3(s) (4)
SiO2+2OH-→ +H2O (5)
+H2O (5)
2Na++ +
+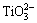 +H2O→Na2TiSiO5(s)+2OH- (6)
+H2O→Na2TiSiO5(s)+2OH- (6)
Na2TiSiO5 contains layers of tetrahedral SiO4 and square pyramidal TiO5 joined at the corners and separated by layers of Na+ ions [26]. Na2TiO3 was in the form of α-Na2TiO3 in this study, and it exhibited the NaCl-type structure with the Na and Ti ions randomly placed in the cation sites. It had a closely packed array of Na and Ti atoms in an octahedral coordination [27]. The bond length of Ti—O calculated by XRD was about 2.250  in Na2TiO3, which was longer than those in rutile (from 1.949 to 1.980
in Na2TiO3, which was longer than those in rutile (from 1.949 to 1.980  ) and Na2TiSiO5 (from 1.695 to 1.990
) and Na2TiSiO5 (from 1.695 to 1.990  ) [28]. This result indicates that the Na2TiO3 structure was more active and metastable.
) [28]. This result indicates that the Na2TiO3 structure was more active and metastable.
The XRD patterns shown in Fig. 4 indicate that more Na2TiSiO5 formed with decreasing the NaOH/tionite mass ratio. When the NaOH/tionite mass ratio was 6:1 in 50% NaOH concentration, Na2TiSiO5 was not detected. According to Eqs. (3) and (5), the NaOH concentration decreased as the hydrothermal conversion proceeded because the reactions consumed NaOH. At a high NaOH concentration of 50%, 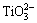 and
and  would combine with Na+ to form Na2TiO3 and Na2SiO3, respectively. At a low NaOH concentration, Na2TiO3 would be unstable or disintegrated, and
would combine with Na+ to form Na2TiO3 and Na2SiO3, respectively. At a low NaOH concentration, Na2TiO3 would be unstable or disintegrated, and 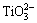 would integrate with
would integrate with  to form Na2TiSiO5, as demonstrated in Eq. (6). The low NaOH concentration is inferred to be the key factor that leaded to the formation of Na2TiSiO5.
to form Na2TiSiO5, as demonstrated in Eq. (6). The low NaOH concentration is inferred to be the key factor that leaded to the formation of Na2TiSiO5.
In addition, the reaction of ilmenite with NaOH solution under oxygen atmosphere is shown as follows [29]:
4FeTiO3+8NaOH+O2→4Na2TiO3+2Fe2O3+4H2O (7)
O2 has an important function in the hydrothermal conversion of ilmenite. O2 could aid the decomposition of FeTiO3 and increase the total conversion of titanium in tionite. When the amount of O2 is low, Reaction (7) will generate Fe3O4 or FeO, which are hardly soluble in NaOH solution and would surround the ilmenite particle to prevent further conversion reactions. However, with enough O2 amount, the generated Fe2O3 underwent a dissolution/precipitation process in NaOH solution which could promote the decomposition of ilmenite. The re-precipitated Fe2O3 had low degree of crystallinity, thus, fewer peaks were shown in the XRD patterns. The solubility of Fe2O3 increases with increasing the reaction temperature and NaOH concentration [30], and the reaction is present as follows:
Fe2O3+2NaOH→ +2Na++H2O (8)
+2Na++H2O (8)
As shown in Fig. 6, a higher oxygen partial pressure led to a higher reaction rate and intense conversion of titanium-based minerals. In concentrated NaOH solutions, an acid–base equilibrium existed [31]:
2OH-→H2O+O2- (9)
Another solubility equilibrium is described as follows [32]:
1/2O2+O2-→ (10)
(10)
When the oxygen partial pressure increased, the quantity of O2- and  clusters increased. They oxidized the iron components in ilmenite and facilitated the breakage and decomposition of Ti-O bonds. In this sense, a certain amount of oxygen must be injected into the autoclave to obtain the complete conversion of titanium-based minerals, especially ilmenite in tionite.
clusters increased. They oxidized the iron components in ilmenite and facilitated the breakage and decomposition of Ti-O bonds. In this sense, a certain amount of oxygen must be injected into the autoclave to obtain the complete conversion of titanium-based minerals, especially ilmenite in tionite.
The following optimal hydrothermal parameters were proposed to obtain the ideal phase of Na2TiO3 and avoid the formation of Na2TiSiO5: NaOH concentration of 50%, NaOH/tionite mass ratio of 4:1, reaction temperature of 240 °C, reaction time of 1 h and oxygen partial pressure of 0.25 MPa. The hydrothermal product obtained under the above optimum hydrothermal conditions, namely hydrothermal product C, had a titanium conversion of 97.2% and its prominent phase of titanium was Na2TiO3.
3.2 Water washing of hydrothermal products and NaOH recirculation
Na+ in the hydrothermal products should be recovered for NaOH recirculation by water washing. Up to now, there was no report on the behaviors of Na2TiSiO5 in water. To further figure out the difference in ion-exchange ability of Na2TiSiO5 and Na2TiO3, the representative hydrothermal products B (main phase is Na2TiSiO5) and C (main phase is Na2TiO3), were washed in water with the water/hydrothermal product mass ratio of 1.5:1 at 55 °C four times. The XRD patterns of the water washed products are shown in Fig. 9. The peaks of Na2TiO3 and Na2SiO3 in hydrothermal product C both disappeared, and the water washed product C remained as an amorphous phase with very weak TiO2 and FeTiO3 peaks, which means that Na2TiO3 had excellent ion-exchange ability in water. However, Na2TiSiO5 in hydrothermal product B remained stable in water washed product B. Further ICP-OES analysis shows that Na+ in Na2TiSiO5 could hardly be leached in water. Hence, the loss of Na+ was greatly attributed to Na2TiSiO5 formation. Just as reported before, Na+ in Na2TiO3 could easily exchange with H+ in water [27]. This exchange was the basis of NaOH recirculation in this process. The ion exchange reaction could be described as follows:
Na2TiO3+xH2O→Na2-xHxTiO3+xNaOH (11)

Fig. 9 XRD patterns of hydrothermal products B and C after water washing
Thus, Na+ in the structure of Na2TiO3 could also be recycled via water washing. The leaching results of Na+ and the removal fractions of other impurities via water washing are shown in Fig. 10. The total recovery of Na+ after washing four times was 97.6%. Moreover, the total removal fractions of Si, Al, and Mn were 78.3%, 93.3% and 29.7%, respectively. The compositional analysis results of the water washed product are listed in Table 2.

Fig. 10 Leaching fractions of Na, Si, Al and Mn at varying water washing times
Table 2 Compositional analysis results of water washed product (mass fraction, %)

Si was the major impurity which could lead to the formation of Na2TiSiO5. Thus, Na2SiO3 in the recycled NaOH solution should be limited. Table 3 indicates that the solubility of Si decreased with the increase of NaOH concentration. The concentrations of NaOH and Si in hydrothermal converted solution (CS) was 735.6 and 4.6 g/L, respectively. This result indicates that Si would not accumulate in the solution when it was reused. Further experiments reveal that residual Si exhibited no effect on the hydrothermal conversion when CS was directly reused without desilication. The results shown in Fig. 10 indicate that only 16.5% of Si remained in CS, and 61.8% of Si was leached in water after water washing. To remove Si impurities from water washed solution (WS), CaO was added when WS was heated at 95 °C, and more than 92% of Si was removed from WS with the precipitation of calcium silicates. After desilication, the concentration of Si in WS was less than 0.6 g/L.
Table 3 Solubility of Si in NaOH solutions with different concentrations (Calculated from solubility of Na2SiO3 in NaOH solutions at 50 °C from Ref. [33])

The NaOH concentrations in the CS and WS obtained from four times of countercurrent washing were about 735.6 and 202.7 g/L, respectively. The moderate NaOH concentration gaps made them both economically and technically feasible to be evaporated to a concentration of 50% (about 762.6 g/L) for reuse in hydrothermal conversion.
3.3 Leaching of titanium by H2SO4 solution for TiO2 preparation
Titanium in the washed solid product could be leached by H2SO4 solution to prepare titanyl sulfate solution for TiO2 production. The effect of temperature on the leaching fraction of titanium was investigated with 20% H2SO4. The results shown in Fig. 11 indicate that a large portion of titanium could be easily leached by 20% H2SO4 at low temperatures for 60 min. The leaching fraction of titanium increased with increasing the reaction temperature. However, the amount of titanium leached at 75 °C decreased after 120 min. This decrease may be due to the hydrolysis of titanium at higher temperature for a long time.

Fig. 11 Effect of leaching temperature and time on titanium leaching fraction
The results shown in Table 4 indicate that higher H2SO4 concentration tended to obtain higher titanium leaching fraction, and 96.7% of titanium could be leached in 40% H2SO4 solution at 55 °C for 60 min. The composition of the prepared titanyl sulfate solution was shown in Table 5. The results indicate that with the exception of Si, most of the other impurities could be leached. Most of the Si was separated from the solution in the form of silica gel remaining as a solid residue under the above conditions.
Table 4 Titanium leaching fraction by different H2SO4 concentrations

The H2SO4 concentration and reaction temperature in this process were much lower than those used for the digestion of ilmenite in the traditional sulfate process (85%-95% H2SO4, 180-200 °C) [10]. The apparent activation energy for the decomposition of titanium intermediates Na2-xHxTiO3 by H2SO4 was reported to be 28.7 kJ/mol, which was much lower than that for the decomposition of ilmenite by H2SO4 (72.6 kJ/mol) [33]. Therefore, the titanium in the washed solid product could be easily leached by 20%-60% H2SO4 solution at 30-75 °C. Due to its good acid-leaching performance of the washed solid product, the waste acid containing about 20% H2SO4 generated from the sulfate process for TiO2 production could be used directly or after concentration in the above acid leaching process.
After concentration, the titanyl sulfate solution was hydrolyzed at boiling temperature, and the obtained hydrolysis product H2TiO3 was then washed and calcinated to prepare TiO2. The XRD pattern and SEM image shown in Fig. 12 indicate that the obtained TiO2 product was well-crystallized rutile with the average sizes of elementary particles of 0.3-0.5 μm. The chemical composition of the obtained product was 99.4% TiO2, 0.037% SiO2, 0.013% ∑Fe, 0.051% MgO, and 0.113% SO3. The product could be used as TiO2 white pigment after post-treatment.
Table 5 Compositional analysis results of prepared titanyl sulfate solution (g/L)


Fig. 12 XRD pattern and SEM image of obtained TiO2 product
4 Conclusions
1) A new process for the recovery of titanium from tionite was proposed. This process comprised of NaOH hydrothermal conversion, water washing, and H2SO4 leaching for TiO2 preparation.
2) Under the optimum hydrothermal conversion conditions, i.e., NaOH concentration of 50%, NaOH/ tionite mass ratio of 4:1, reaction temperature of 240 °C, reaction time of 1 h, and oxygen partial pressure of 0.25 MPa, about 97.2% of titanium in tionite was converted and Na2TiO3 was the main phase of titanium. The increase of NaOH concentration and NaOH/tionite mass ratio in hydrothermal conversion prevented the formation of the unwanted product Na2TiSiO5.
3) About 97.6% of Na+ could be recycled after washing the optimum hydrothermal products with water four times. The hydrothermal converted solution could be reused without desilicication. More than 92% of Si was removed from the water washed solution by adding CaO, and the obtained NaOH solution could be reused after concentration.
4) Titanium in the water washed product was easily leached by 20%-60% H2SO4 solution at 30-75 °C, forming titanyl sulfate solution to further produce TiO2. The optimum recovery of titanium was up to 96.7%.
References
[1] SUI Li-li, ZHAI Yu-chun. Reaction kinetics of roasting high-titanium slag with concentrated sulfuric acid [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(3): 848-853.
[2] TIAN Cong-xue, HUANG Shuang-hua, YANG Ying. Anatase TiO2 white pigment production from unenriched industrial titanyl sulfate solution via short sulfate process [J]. Dyes and Pigments, 2013, 96(2): 609-613.
[3] LIU Chen, LI You-ji, XU Peng, LI Ze-shi, ZENG Meng-xiong. Preparation and improved photocatalytic activity of ordered mesoporous TiO2 by evaporation induced self-assembly technique using liquid crystal as template [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(4): 1072-1078.
[4] MIDDLEMAS S, FANG Z Z, FAN P. A new method for production of titanium dioxide pigment [J]. Hydrometallurgy, 2013, 131-132: 107-113.
[5] CHEN De-sheng, ZHAO Long-sheng, QI Tao, HU Guo-ping, ZHAO Hong-xin, LI Jie, WANG Li-na. Desilication from titanium- vanadium slag by alkaline leaching [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(10): 3076-3082.
[6] GAZQUEZ M J, BOL VAR J P, GARCIA T R, VACA F. Physicochemical characterization of raw materials and co-products from the titanium dioxide industry [J]. Journal of Hazardous Materials, 2009, 166(2-3): 1429-1440.
[7] GAZQUEZ M J, MANTERO J, BOLIVAR J P, GARCIA T R, VACA F, LOZANO R L. Physico-chemical and radioactive characterization of TiO2 undissolved mud for its valorization [J]. Journal of Hazardous Materials, 2011, 191(1-3): 269-276.
[8] DONDI M, GUARINI G, RAIMONDO M, ZANELLI C, FABBRICHE D D, AGOSTINI A. Recycling the insoluble residue from titania slag dissolution (tionite) in clay bricks [J]. Ceramics International, 2010, 36(8): 2461-2467.
[9] HAJJAJI W, COSTA G, ZANELLI C, RIBEIRO M J, SEABRA M P, DONDI M, LABRINCHA J A. An overview of using solid wastes for pigment industry [J]. Journal of the European Ceramic Society, 2012, 32(4): 753-764.
[10] CHEN De-bin. Practical questions and qnswers in the production of titanium doxide from sulfate process [M]. Beijing: Chemical Industry Press, 2009. (in Chinese)
[11] CONTRERAS M, GAZQUEZ M J, GARCIA-DIAZ I, ALGUACIL F J, LOPEZ F A, BOLIVAR J P. Valorisation of waste ilmenite mud in the manufacture of sulphur polymer cement [J]. Journal of Environmental Management, 2013, 128: 625-630.
[12] LABRINCHA J A, MARQUES J I, HAJJAJI W, SENFF L, ZANELLI C, DONDI M, ROCHA F. Novel inorganic products based on industrial wastes [J]. Waste and Biomass Valorization, 2014, 5(3): 385-392.
[13] BELARDI G, PIGA L, QUARESIMA S, SHEHU N. Application of physical separation methods for the upgrading of titanium dioxide contained in a fine waste [J]. International Journal of Mineral Processing, 1998, 53(3): 145-156.
[14] JIANG Peng-gang. Method for recycling titanium from sulfuric acid method titanium white acidolysis residue: China, 201210248845 [P]. 2012-11-07. (in Chinese)
[15] LIU Bu-jin, ZHU Quan-fang, CAI Ping-xiong, CHEN Jian, SUN Run-fa, ZENG Xiao-lin. Recycling method for producing acidolysis slag in titanium dioxide with sulfuric acid method: China, 201210095177 [P]. 2012-07-25. (in Chinese)
[16] LIU Qing, YANG Sheng-hai, CHEN Yong-ming, HE Jing, XUE Hao-tian. Selective recovery of lead from zinc oxide dust with alkaline Na2EDTA solution [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(4): 1179-1186.
[17] YANG Quan-cheng, MA Shu-hua, ZHENG Shi-li, ZHANG Ran. Recovery of alumina from circulating fluidized bed combustion Al-rich fly ash using mild hydrochemical process [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(4): 1187-1195.
[18] KATSUKI H, FURUTA S, SHIRAISHI A, KOMARNENI S. Porous mullite honeycomb by hydrothermal treatment of fired kaolin bodies in NaOH [J]. Journal of Porous Materials, 1996, 2(4): 299-305.
[19] ZHANG Yong-jie, QI Tao, ZHANG Yi. A novel preparation of titanium dioxide from titanium slag [J]. Hydrometallurgy, 2009, 96(1-2): 52-56.
[20] MENG Fan-cheng, LIU Ya-hui, CHU Jing-long, WANG Wei-jing, QI Tao. Structural control of Na2TiO3 in pre-treating natural rutile ore by alkali roasting for TiO2 production [J]. The Canadian Journal of Chemical Engineering, 2014, 92(8): 1346-1352.
[21] XUE Tian-yan, WANG Li-na, QI Tao, CHU Jing-long, QU Jing-kui, LIU Chang-hou. Decomposition kinetics of titanium slag in sodium hydroxide system [J]. Hydrometallurgy, 2009, 95(1-2): 22-27.
[22] KOSTOV K V, FERDOV S, KALVACHEV Y, MIHAILOVA B, PETROV O. Hydrothermal synthesis of microporous titanosilicates [J]. Microporous and Mesoporous Materials, 2007, 105(3): 232-238.
[23] ZHANG Hai, XU Hong-bin, ZHANG Xiao-fei, ZHANG Yang, ZHANG Yi. Pressure oxidative leaching of Indian chromite ore in concentrated NaOH solution [J]. Hydrometallurgy, 2014, 142: 47-55.
[24] BYRAPPA K, YOSHIMURA M. Handbook of hydrothermal technology [M]. New York: William Andrew Publishing, 2001.
[25] PARK H, ENGLEZOS P. Osmotic coefficient data for Na2SiO3 and Na2SiO3–NaOH by an isopiestic method and modeling using Pitzer's model [J]. Fluid Phase Equilibria, 1998, 153(1): 87-104.
[26] FERDOV S. A comparative rietveld refinement study of natisite prepared in different morphology [J]. Journal of Chemical Crystallography, 2013, 43(8): 443-447.
[27] LIU Ya-hui, ZHAO Wei, WANG Wei-jing, YANG Xuan, CHU Jing-long, XUE Tian-yan, QI Tao, WU Jing-yi, WANG Chun-ru. Study on the transformation from NaCl-type Na2TiO3 to layered titanate [J]. Journal of Physics and Chemistry of Solids, 2012, 73(3): 402-406.
[28] PENG G W, LIU H S. FT-IR and XRD characterization of phase transformation of heat-treated synthetic natisite (Na2TiOSiO4) powder [J]. Materials Chemistry and Physics, 1995, 42(4): 264-275.
[29] AMER A M. Alkaline pressure leaching of mechanically activated Rosetta ilmenite concentrate [J]. Hydrometallurgy, 2002, 67(1-3): 125-133.
[30] ISHIKAWA K, YOSHIOKA T, SATO T, OKUWAKI A. Solubility of hematite in LiOH, NaOH and KOH solutions [J]. Hydrometallurgy, 1997, 45(1): 129-135.
[31] DUAN Shu-zhen, QIAO Zhi-yu. Molten salt chemistry—Principle and application [M]. Beijing: Metallurgical Industry Press, 1990. (in Chinese)
[32] SUN Zhi, ZHANG Yi, ZHENG Shi-Li, ZHANG Yang. A new method of potassium chromate production from chromite and KOH-KNO3-H2O binary submolten salt system [J]. AIChE Journal, 2009, 55(10): 2646-2656.
[33] BAKER C L, JUE L R, WILLS J H. The system Na2O-SiO2-H2O at 50, 70 and 90° [J]. Journal of the American Chemical Society, 1950, 72(12): 5369-5382.
[34] WANG Wei-jing, XUE Tian-yan, WANG Dong, QI Tao. Kinetic study on sulfuric acid dissolution of NaxH2-xTiO3 from sodium hydroxide molten method [J]. Advanced Materials Research, 2014, 881-883: 1545-1548.
孟凡成1,2,3,薛天艳1,2,刘亚辉1, 2,张国之1,2,3,齐 涛1,2
1. 中国科学院 过程工程研究所 湿法冶金清洁生产技术国家工程实验室,北京 100190;
2. 中国科学院 过程工程研究所 绿色过程与工程院重点实验室,北京 100190;
3. 中国科学院大学,北京 100049
摘 要:提出一种从黑泥中回收利用钛的新工艺,该工艺包括NaOH水热转化、水洗和H2SO4浸出制备TiO2。在优化的反应条件下,即NaOH溶液浓度为50%(质量分数)、NaOH/黑泥质量比为4:1、反应温度为240 °C、反应时间为1 h和氧气分压为0.25 MPa,钛转化率可达97.2%,主要含钛产物是Na2TiO3。非目标产物Na2TiSiO5在水洗中保持稳定,在水热反应中提高NaOH浓度可以抑制Na2TiSiO5的生成。水热产物经过水洗后,97.6%的Na+可以回收。含有NaOH的溶液经过浓缩之后可以回用。在较低温度下,水洗物料中96.7%的钛能被较低浓度的硫酸浸出得到钛液。利用所得钛液进一步制备合格TiO2产品。
关键词:黑泥;钛回收;NaOH水热转化;水洗;H2SO4浸出
(Edited by Mu-lan QIN)
Foundation item: Project (51090380) supported by the National Natural Science Foundation of China; Projects (2013CB632604, 2013CB632601) supported by the National Basic Research Program of China; Project (51125018) supported by the National Science Foundation for Distinguished Young Scholars of China; Project (KGZD-EW-201-2) supported by the Key Research Program of the Chinese Academy of Sciences; Projects (51374191, 51402303) supported by the Natural Science Foundation for the Youth, China
Corresponding author: Tian-yan XUE; Tel: +86-10-82544848; E-mail: tyxue@ipe.ac.cn
DOI: 10.1016/S1003-6326(16)64247-4

