
Influence of potassium pyrophosphate in electrolyte on coated layer of
AZ91 Mg alloy formed by plasma electrolytic oxidation
Jin-Young CHO, Duck-Young HWANG, Dong-Heon LEE, Bongyoung YOO, Dong-Hyuk SHIN
Department of Metallurgy and Materials Science, Hanyang University, Ansan,426-791, Korea
Received 18 June 2008; accepted 10 March 2009
Abstract: The effect of potassium pyrophosphate in the electrolyte on plasma electrolytic oxidation (PEO) process for AZ91 Mg alloy was investigated. The morphologies and chemical compositions of the coating layer on the AZ91 Mg alloy were evaluated and corrosion resistance was also estimated by potentiodynamic polarization analysis. The coating layer on AZ91 Mg alloy coated from the Bath 2 containing 0.03 mol/L of potassium pyrophosphate for 360 s exhibited considerably dense structure and contained 11%-18% (mass fraction) of phosphorous. The higher content of phosphorous of coating layer coated from Bath 2 could be detected at the bottom of oxide layer, which strongly implied that the phosphorous ion might be concentrated at the barrier layer. Corrosion potential of coating layer of AZ91 Mg alloy increased and corrosion current density decreased with increasing the concentration of potassium pyrophosphate. The polarization resistance (Rp) of coating layer of AZ91 Mg alloy coated from Bath 2 was 4.65×107 Ω/cm2, which was higher than that (Rp=3.56×104 Ω/cm2) of the sample coated from electrolyte without potassium pyrophosphate. The coating layer coated from Bath 2 containing 0.03 mol/L potassium pyrophosphate exhibited the best corrosion resistance.
Key words: plasma electrolytic oxidation; magnesium alloy; phosphorous; corrosion resistance
1 Introduction
Magnesium alloys have excellent physical and mechanical properties, such as high strength, low density and good castability. So they have been used in a lot of fields, such as mobile, aerospace, construction, and computer, Unfortunately, magnesium alloys have poor corrosion resistance, especially in acidic environments and in salt-water condition[1]. Conventional surface treatment methods such as cremating, non-cromating process are not satisfied. These processes have the disadvantages of low corrosion resistance and wastewater problems. Therefore, it is worth developing a new process of surface treatment to solve these problems. Plasma electrolytic oxidation (PEO) process is a relatively new surface treatment method to form oxide layer on magnesium alloys using an environmentally friendly process[2]. An important feature of PEO process is a plasma discharge that occurs at the metal/electrolyte interface when the applied voltage exceeds a certain critical breakdown value. The constituents of surface oxides contribute from substrate and the anodic complexes from the electrolyte. The structures of PEO process depend on various processing conditions, including chemical composition and concentration of electrolyte, current density, and alloy composition of substrate[3-4]. Especially, the chemical composition of the electrolyte exerts a considerable influence on the property and formation of effective ceramic coating for Mg alloy. Moreover, some additives, such as phosphate, fluoride, and borate are commonly used[5]. In this study, the effect of potassium pyrophosphate in the electrolyte on PEO process for AZ91 Mg alloy was investigated. The morphologies and chemical compositions of the coating layer on the AZ91 Mg alloy were evaluated and corrosion resistance was estimated by potentiodynamic polarization analysis.
2 Experimental
Commercial AZ91 ingots were used as substrate in this study. AZ91 Mg alloy plates with 30 mm×50 mm×2 mm were ground to a grit of 1 000 by SiC paper, then rinsed with de-ionized water, ultrasonically cleaned in ethanol, and finally dried in warm air. PEO process was conducted at 20 kW. The equipment for PEO has a glass-vessel container with a sample holder as the electrolyte cell, a stainless steel used as the cathode, and stirring and cooling system. Table 1 lists the chemical compositions of electrolyte for PEO process. The current density was controlled constant at 50 mA/cm2. Temperature of electrolyte was maintained at 20-30 ℃ during PEO process. The morphology and chemical compositions of the oxide layer were observed using a scanning electron microscope (TESCAN 510). Potentiodynamic polarization to evaluate corrosion resistance of the coated AZ91 Mg alloy was carried out using Reference 600 Potentiostat (Gamry Instruments). As we know, Mg alloy is sensitive to occur pitting corrosion in electrolyte or environment containing Cl-.
Table 1 Electrolyte compositions for PEO process in AZ91 Mg alloy
3 Results and discussion
Fig.1 shows the surface morphology of oxide layer in AZ91 Mg alloy coated from the electrolyte with a variation of the concentration of the potassium pyrophosphate for 360 s. As shown in Fig.1, coating layer of the AZ91 Mg alloy exhibited the typical surface morphology for PEO process regardless of the kinds of electrolyte. During PEO process, the metal surface was progressively covered with an oxide layer. The oxide layer showed surface morphology of dense crater-like microstructures with some round-shape shrinkage pores observed in the crater centers. Surface morphology with small-pore-size oxide layer in Bath 1 was observed (Fig.1(a)). The pore size on the surface of coating layer increased with increasing the concentration of the potassium pyrophosphate in the electrolyte. The coating layer coated from Bath 2 exhibited the most small size pores on surface.

Fig.1 Surface morphologies of oxide layer of AZ91 Mg alloy in electrolyte containing various concentrations of potassium pyrophosphate coated for 360 s: (a) Bath 1; (b) Bath 2; (c) Bath 3; (d) Bath 4
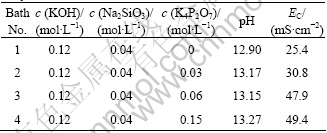
The cross-sectional images of layer coated from the electrolytes with and without potassium pyrophosphate are shown in Fig.2. There were many micro pores and some micro cracks on surfaces of coating layer. These pores and cracks were neither connected with each other nor perforated through the whole coating layer. Previous studies showed that pores in the coating layer were formed by gas bubbles thrown out of micro-arc discharge channels while cracks were resulted from thermal stress due to rapid solidification of molten oxide in the relatively cool electrolyte[2]. In the ordinary way, the coating layer formed on the AZ91 Mg alloy applied in the PEO process was composed of two different layers: an outer porous layer and an inner barrier layer, which is a very thin layer with thickness of 100-300 nm[4-5]. The structure of coating layer was different with the variation of the concentration of potassium pyrophosphate. The sample coated from Bath 2 with potassium pyrophosphate exhibited relative uniform structure, compared with the other conditions (Fig.2(b)).
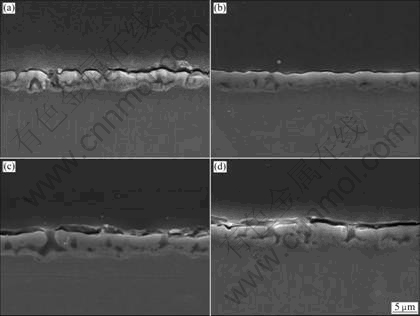
Fig.2 Cross-sectional images of oxide layer of AZ91 Mg alloy in electrolyte containing various concentrations of potassium pyrophosphate coated for 360 s: (a) Bath 1; (b) Bath 2; (c) Bath 3; (d) Bath 4
Fig.3 show the changes of the coating thickness as a function of the coating time coated from electrolytes with a variation of the potassium pyrophosphate. It was observed that coating thickness increased with increasing coating time regardless of kinds of electrolytes. However, the exfoliation between the coated layer and the matrix occurred in the Bath 3 and Bath 4 over 600 s.

Fig.3 Variation of thickness of PEO coatings formed in electrolytes containing various concentrations of potassium pyrophosphate
The growth rate of coating layer on the AZ91 Mg alloy was same treated until 360 s with and without potassium pyrophosphate. Thickness of coating layer treated until 360 s was measured to be ~3.8 μm. The growth rate of coating layer on AZ91 Mg alloy coated after 360 s exhibited far different with kinds of electrolytes. The growth rate of coating layer coated from Bath 3 is faster than that of the other conditions. However, the surface roughness of the coating layer increased due to the increase in the diameters of the pores on the surface of coating layer. Other researchers reported that the coating layer had an influence on the surface roughness[6-7].
From the result of the EDS analysis of the oxide layer on the cross-section of AZ91 Mg alloy, the component of the oxide layer coated from the electrolyte containing potassium pyrophosphate consisted of oxygen, phosphorous, silicon, magnesium, and aluminum, which strongly supported that phosphorous was successfully incorporated in oxide layer (Fig.4(a)). The contents of phosphorous ions in the oxide layer coated from the electrolytes for 360 s increased with increasing the concentration of potassium pyrophosphate in the electrolyte. The content of phosphorous ion existed in the coating layer coated from Bath 4 was detected to be about 30%. In the case of the sample coated for 360 s, the contents of phosphorous ions existed in the porous layer and barrier layer were measured to be 11% and 18%, respectively (Fig.4(b)). In the other words, the higher content of phosphorous could be detected at the bottom of oxide layer, which strongly implied that the phosphorous ion might be concentrated at the barrier layer.
Fig.4 Energy-dispersive plot of oxide layer on AZ91 Mg alloy treated from Bath 2 for 360 s
Corrosion resistance of the coating in the AZ91 Mg alloy was evaluated by electrochemical potentiodynamic polarization in 3.5% NaCl solution. Fig.5 exhibits the potentiodynamic polarization curve of coating layer of AZ91 Mg alloy coated from the electrolyte with a variation of the concentration of potassium pyrophosphate. It is well known that corrosion potential and current density of coated samples were often used to characterize corrosion protective property of the coating [8-9]. In general, it was reported that the high corrosion potential and low corrosion current density of the coating exhibited a low corrosion rate and a good corrosion resistance[8]. Corrosion potential of coating layer of AZ91 Mg alloy increased and corrosion current density decreased with increasing the concentration of potassium pyrophosphate. In the case of the sample coated from Bath 4 with the high content of phosphorous ion, corrosion potential and corrosion current density were similar to the sample coated from Bath 1 without potassium pyrophosphate. Especially, corrosion current density of coating layer of AZ91 Mg alloy coated from Bath 2 containing 0.03 mol/L potassium pyrophosphate was three orders lower than that of coating layer of AZ91 Mg alloy coated from Bath 1 without potassium pyrophosphate. The corrosion potential (φcorr), corrosion current density (Jcorr), and anodic/cathodic Tafel slopes (ba and bc) were derived from these data. Based on the approximately linear polarization behavior near OCP, the polarization resistance (Rp) values were determined from Stern-Geary equation[10]:
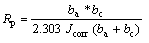 (1)
(1)
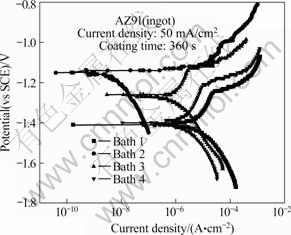
Fig.5 Potentiodynamic polarization curves of oxide layer on AZ91 Mg alloy coated from electrolyte containing various concentrations of potassium pyrophosphate
The results of potentiodynamic polarization are summarized in Table 2. The polarization resistance (Rp) of coating layer of AZ91 Mg alloy coated from Bath 2 was 4.65×107 Ω/cm2, which was higher than that (Rp=3.56×104 Ω/cm2) of the sample coated from electrolyte without potassium pyrophosphate. It was reported that some additives such as phosphate and fluoride were very commonly used for the formation of stable products (Mg3(PO4)2 or MgF2)[7]. These stable products could act as barrier layer preventing magnesium substrate from corrosion in most corrosive intermediate. Phosphate was useful to enhancing the corrosion resistance of inner barrier layer of PEO process. The corrosion resistance of all the samples can be ranked as: Bath 2>Bath 3>Bath 4>Bath 1. The coating layer formed on the surface of AZ91 Mg alloy in electrolyte containing phosphorous ions fabricated by PEO process provided effective corrosion protection to substrate in electrolyte containing Cl- ions.
Table 2 Results of potentiodynamic corrosion tests of four samples in 3.5% NaCl solution

4 Conclusions
The effect of potassium pyrophosphate in electrolyte on PEO process for AZ91 Mg alloy was investigated. The pore size on the surface of coating layer increased with increasing concentration of the potassium pyrophosphate in the electrolyte. The structure of coating layer was different with the variation of the concentration of potassium pyrophosphate. The sample coated from Bath 2 with potassium pyrophosphate exhibited relatively uniform structure, compared with the other conditions. The contents of phosphorous ions in the oxide layer coated from the electrolytes for 360 s increased with increasing the concentration of potassium pyrophosphate in the electrolyte. Corrosion potential of coating layer of AZ91 Mg alloy increased and corrosion current density decreased with increasing the concentration of potassium pyrophosphate. The polarization resistance (Rp) of coating layer of AZ91 Mg alloy coated from Bath 2 was 4.65×107 Ω/cm2, which was higher than that (Rp=3.56×104 Ω/cm2) of the sample coated from electrolyte without potassium pyrophosphate. Phosphate was useful to enhancing the corrosion resistance of inner barrier layer of PEO process. The coating layer coated from Bath 2 containing 0.03 mol/L potassium pyrophosphate exhibited the best corrosion resistance.
Acknowledgement
This research was supported by a grant from the Center for Advanced Materials Processing (CAMP) of the 21st Century Frontier R&D Program funded by the Ministry of Knowledge Economy (MKE), Korea. This work was also supported by the Korea Science and Engineering Foundation (No. 2009-0079807).
References
[1] LIN C S, FU Y C. Characterization of anodic films on AZ31 magnesium alloys in alkaline solutions containing fluoride and phosphate anions [J]. Electrochem Soc, 2006, 153: B417-B424.
[2] YEROKIN L, NIE X, LEYAND A, MATTEWS A, DOWEY S J. Plasma electrolysis for surface engineering: Review [J]. Sruf Coat Technol, 1999, 122: 73-93.
[3] LINAG J, GUO B, TIAN J, LIU H, ZHOU J, LIU W, XU T. Effects of NaAlO2 on structure and corrosion resistance of microarc oxidation coatings formed on AM60B magnesium alloy in phosphate-KOH electrolyte [J]. Surf Coat Technol, 2005, 199: 121-126.
[4] KHASELEV O, WEISS D, YAHALOM J. Structure and composition of anodic films formed on binary Mg-Al alloys in KOH-aluminate solutions under continuous sparking [J]. Corros Sci, 2001, 43: 1295-1307.
[5] BIRSS V, XIA S, YUE R, Jr RATEICK R G. Characterization of oxide films formed on Mg-based WE43 alloy using AC/DC anodization in silicate solutions [J]. Electrochem Soc, 2004, 151: B1-B10.
[6] GUO H, AN M Z. Growth of ceramic coatings on AZ91D magnesium alloys by micro-arc oxidation in aluminate-fluoride solutions and evaluation of corrosion resistance [J]. App Surf Sci, 2005, 246: 229-238.
[7] DUAN H, YAN A, WANG F. Growth process of plasma electrolytic oxidation films formed on magnesium alloy AZ91D in silicate solution [J]. Electrochim Acta, 2007, 52: 5002-5009.
[8] SHI Z, SONG G, ATRENS A. Influence of the b phase on the corrosion performance of anodised coatings on magnesium- aluminium alloy [J]. Corros Sci, 2005, 47: 2760-2777.
[9] RUDD L, BERSLIN C B, MANSFELD F. The corrosion protection afforded by rare earth conversion coatings applied to magnesium [J]. Corros Sci, 2000, 42: 275-288.
[10] STERN M, GEARY A L. Electrochemical polarization [J]. Elcetrochem Soc, 1957, 104: 56-63.
Corresponding author: Dong-Hyuk SHIN; Tel: +82-31-400-5224, E-mail: dhshin@hanyang.ac.kr
DOI: 10.1016/S1003-6326(08)60358-1
(Edited by YANG Bing)