J. Cent. South Univ. Technol. (2008) 15: 438-442
DOI: 10.1007/s11771-008-0082-z
Effect of catalyst on structure of (PEO)8LiClO4-SiO2 composite polymer electrolyte films
PAN Chun-yue(潘春跃), ZHANG Qian(张 倩), FENG Qing(冯 庆),
GAO Jin-huan(高金环), ZHAO You-man(赵悠曼)
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, china)
Abstract: (PEO)8LiClO4-SiO2 composite polymer electrolytes(CPEs) were prepared by in-situ reaction, in which ethyl-orthosilicate (TEOS) was catalyzed by HCl and NH3·H2O, respectively. The ionic conductivity, the contact angle and the morphology of inorganic particles in the CPEs were investigated by AC impedance spectra, contact angle method and TEM. The conductivities of acid-catalyzed CPE and alkali-catalyzed CPE are 2.2×10-5 and 1.1×10-5 S/cm respectively at 30 ℃. The results imply that the catalyst plays an important role in the structure of in-situ preparation of SiO2, and influences the surface energy and conductivity of CPE films directly. Meanwhile, the ionic conductivity is related to the surface energy.
Key words: PEO(polyethylene oxide); SiO2; composite polymer electrolyte; conductivity; catalytic reaction
1 Introduction
Ionic conducting polymer electrolytes consisting of lithium salts and poly capability ethylene oxide with high relative molecule mass have been extensively studied[1]. Compared with their liquid counterparts, polymer electrolytes have advantages of longer shelf-life, better leak-proof capability, and easy fabrication into a wide variety of shapes and sizes[2].
Recently, composite solid polymer electrolytes (CSPEs) made of an inorganic component and a conventional salt-in-polymer electrolyte have received considerable attention due to the suppression of PEO crystallization[3-10], the enhancement of the mechanical properties[11-14] and the conductivity[15-16] and Li+ transference numbers[17]. All these parameters are important for successful application of these SPEs in secondary lithium batteries.
Composite polymer electrolytes(CPEs) can be prepared by different ways, but all techniques can be placed into two major groups: the inorganic component is preformed and then mixed with a polymer; the inorganic component is formed in situ within a polymer electrolyte. The former method can be represented by the incorporation of inorganic fillers (TiO2, Al2O3, SiO2, etc) or china clay platelets in polymers. The latter method is represented by formation of metal oxides within a polymeric system, thus fresh interfaces between inorganic and polymer components are developed. According to a number of reports, the interface between the inorganic and polymeric components plays an important role in enhancement of the CSPE properties. In-situ formation of the inorganic component can result in nanoparticles or interpenetrating networks (or both)[18-19], yet the morphology of the inorganic component depends on its fraction and reaction conditions. The forces existing between organic and inorganic components can also vary between physical sorption, when organic and inorganic components are merely intimately mixed[20], and chemical bonding[18-19, 21].
(PEO)8LiClO4-SiO2 composites polymer electrolytes were prepared by in-situ reaction that involves the simultaneous formation of the polymer network and inorganic nanoparticles in this work. Electrochemical properties were measured by electrochemical impedance spectroscopy(EIS). (PEO)8LiClO4-SiO2 composites polymer electrolyte films were characterized by measuring the contact angles of several liquids on their surfaces. Surface energies were calculated from their contact angle using the Young’s equation and standard computational methods[22]. The morphologies of the inorganic particles in the composite polymer were examined by TEM.
2 Experimental
2.1 Materials
Polyethylene oxide (PEO, relative molecule mass of PEO is 600 000, Aldrich) was dried at 50 ℃ for 24 h under vacuum before being used. LiClO4 (analytical grade) was dried at 120 ℃ for 24 h under vacuum to remove crystal-water, and acetonitrile (analytical grade,99.5%) was used as solvent in the film-casting process and tetraethoxy silane(TEOS) was used as precursor for the inorganic filler.
2.2 Preparation of composite polymer electrolyte films
The required amounts of PEO and LiClO4 were dissolved in acetonitrile and the mixture was stirred at room temperature for 3 h until a homogeneous solution was obtained. Then the mixed solution composed of TEOS, ethanol and catalyst (mass ratio of TEOS to ethanol=1?10) was added, and the pH was adjusted both by HCl and NH3·H2O to 4 and 9 respectively, and the solution was stirred at 55 ℃ for about 3 h until a homogeneous mixture was obtained, and (PEO)8LiClO4- SiO2 composite polymer electrolyte was formed. The mass ratio of PEO to Li was fixed to be 8?1 for all the samples, and the content of SiO2 in the sample was fixed to be 10%.
The films were achieved by casting the solution on the Teflon dish in air at room temperature to allow the evaporation of acetonitrile, and the films were dried under vacuum at 50 ℃ for 48 h to remove the rest of residual solvent, then composite polymer electrolyte films were made.
2.3 Measurements of composite polymer electrolyte films
AC impedance spectra of the composite polymer electrolyte films were measured by use of CHI660 electrochemical workstation (Chenhua, Shanghai) between 1 Hz and 100 000 Hz with 30 mV amplitude. The contact angles of four kinds of testing liquids on CSPEs were measured and the surface energies were calculated. The morphologies of the inorganic particles in the composite polymer were examined by Tecnain G2 20 TEM.
3 Results and discussion
3.1 Ionic conductivity
AC impedance spectra of the composite polymer electrolyte films were measured. Fig.1 shows the effect of SiO2 contents on conductivity of composite polymer electrolytes at 30 ℃. The data show that the ionic conductivities of both alkali-catalyzed CPE and acid- catalyzed CPE with SiO2 10% (mass fraction) have maximum values of 1.1×10-5 and 2.2×10-5 S/cm at 30 ℃, respectively. The ionic conductivity of alkali- catalyzed CSPE is smaller than that of acid-catalyzed CSPE when the content of SiO2 is less than 14%.
3.2 Contact angle and surface energy analysis
In order to obtain more direct quantitative informa-
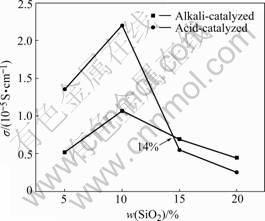
Fig.1 Effect of SiO2 content on conductivity of composite polymer electrolytes at 30 ℃
tion about the CSPE surfaces and the differences between them with different SiO2 contents, surface energies were calculated from their contact angle measurements using the Young’s equation and standard computational methods.
Young’s equation describes the relationship among solid surface energy γS, liquid surface energy γL, solid- liquid surface free energy γSL and contact angle θ.
γS-γSL=γLcos θ (1)
The solid or liquid surface energy is separated into Lifshitz-van der Waals and acid–base contributions, where γLW designates Lifshitz-van der Waals interactions, γAB designates such acid–base interaction as hydrogen bonding, and γ+ and γ- refer to proton and electron donating character, respectively.
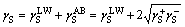 (2)
(2)
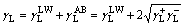 (3)
(3)
The relationship among solid-liquid surface free energy γSL, solid surface energy γS and liquid surface energy γL can be described as
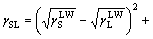
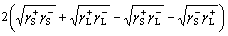 (4)
(4)
Inserting Eqns.(2)-(4) into Eqn.(1), we have
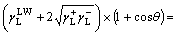
 (5)
(5)
We have four sets of contact angles of liquids on CSPEs with known 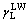 ,
, 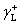 and
and 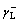 (Table 1), so the surface parameters of CSPEs (
(Table 1), so the surface parameters of CSPEs (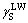 ,
, 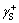 and
and 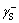 ) can be gotten.
) can be gotten.
Table 1 Surface free energy parameters of test liquids γ/(mJ?m-2)
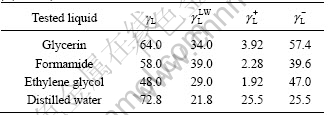
Contact angle data of tested liquids on the surface of electrolyte are listed in Table 2.
Table 2 Contact angle data of tested liquids on surface of electrolyte θ/(°)
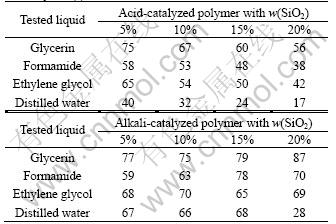
According to Eqn.(5), we calculated the surface energy of CSPE. Fig.2 shows the surface energy with different contents of SiO2. The data show that the surface energy of alkali-catalyzed CSPE has a maximum value of 55.93 mJ/m2 and a minimum value of 33.56 mJ/m2; the surface energy of acid-catalyzed CSPE has a maximum value of 77.45 mJ/m2 and a minimum value of 8.69 mJ/m2. The surface energy of alkali-catalyzed CSPE is higher than that of acid-catalyzed CSPE when SiO2 content is less than 12%. The surface energy of alkali- catalyzed CSPE is smaller than that of acid-catalyzed CSPE when SiO2 content is more than 12%.
We can get the conclusion that the ionic conductivity is related to the surface energy. The larger the surface energy, the smaller the ionic conductivity.
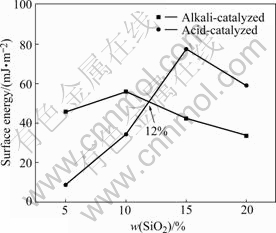
Fig.2 Effect of SiO2 content on surface energy of composite polymer electrolytes
3.3 TEM measurements
In order to observe the difference of (PEO)8LiClO4- SiO2 composite polymer electrolytes catalyzed by HCl and NH3·H2O respectively, the TEM images of the inorganic particles in composite polymer electrolytes after reaction for different time are shown in Fig.3 and Fig.4.

Fig.3 TEM image of SiO2 in alkali-catalyzed (PEO)8LiClO4- SiO2 after reaction for 0.5 h
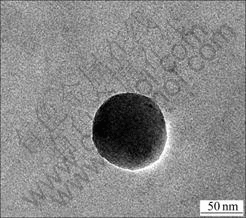
Fig.4 TEM image of SiO2 in alkali-catalyzed (PEO)8LiClO4- SiO2 after reaction for 1 h
There is a round intermediate SiO2 particle with core-shell structure in Fig.3. The core is PEO/LiClO4 and the shell is SiO2. The size of SiO2 particles is about 100 nm. In Fig.4, it is noted that no more core-shell structure in the bulk but solid SiO2 particles. It is possible to see that the filler is well dispersed in the CPE.
Many researchers have studied the influence of catalysts on the hydrolysis-condensation of TEOS. The speed of hydrolysis is faster than that of condensation using alkali catalyst. TEOS hydrolyzes completely in multidimensional direction and forms a shot-chain interpenetrating structure in CPE associated with PEO chain. The intermediate particles with core-shell structure were formed (Fig.3). After 1 h, PEO chain was surrounded by SiO2 network and solid particles formed. The interface between in-situ SiO2 particles and CPE is obvious (Fig.4).
Fig.5 shows TEM image of acid-catalyzed CPE film after reaction for 0.5 h. It is noted that the intermediate particles here are different from Fig.3. The size of particles is 1-2 μm, which is 10-20 times as large as that of alkali-catalyzed SiO2. They are not SiO2 particles but SiO2-PEO/LiClO4 interpenetrating networks. Fig.6 shows TEM image of acid-catalyzed CPE film after reaction for 1 h. It is possible to see that the filler is well dispersed in the CPE. The shape of SiO2 particles in acid-catalyzed CPE is not so round as that in alkali-catalyzed CPE. After 1 h, the size of acid-catalyzed SiO2 particles is 50-100 nm.
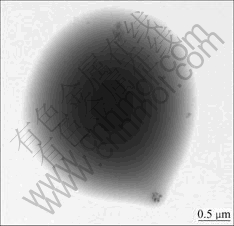
Fig.5 TEM image of SiO2 in acid-catalyzed (PEO)8LiClO4- SiO2 after reaction for 0.5 h

Fig.6 TEM image of SiO2 in acid-catalyzed (PEO)8LiClO4- SiO2 after reaction for 1 h
The speed of TEOS hydrolysis is slower than that of condensation using acid catalyst. TEOS hydrolyzes slowly in CPE associated with PEO chain. The intermediate particles are in big size (Fig.5). After 1 h, the SiO2 particles become smaller because of SiO2 and PEO networks shrinking. The interface between SiO2 and CPE in Fig.6 is not as obvious that of alkali-catalyzed CPE. The compatibility of SiO2 particles in acid- catalyzed CPE is better than that in alkali-catalyzed CPE.
According to the above analysis, different catalysts influence the formation of SiO2 in CPE. The size, the shape, the interface between inorganic filler and CPE are different. All these factors influence ionic conductivity. The ionic conductivity of acid-catalyzed CPE is larger than that of alkali-catalyzed CPE because the compatibility of acid-catalyzed CPE is better than alkali-catalyzed CPE.
4 Conclusions
1) The surface energy of alkali-catalyzed CSPE is higher than that of acid-catalyzed CSPE when SiO2 content is less than around 12%.
2) Compatibility of acid-catalyzed CPE is better than that of alkali-catalyzed CPE.
3) The ionic conductivity is related to the surface energy. The larger the surface energy, the smaller the ionic conductivity. The ionic conductivity of acid- catalyzed CPE is larger than that of alkali-catalyzed CPE, the conductivities of acid-catalyzed CPE and alkali- catalyzed CPE are 2.2×10-5 S/cm and 1.1×10-5 S/cm respectively at 30 ℃.
References
[1] SCROSATI B. Applications of electroactive polymers[M]. London: Chapman and Hall, 1993.
[2] ARMAND M B, CHABAGNO J M, DUCLOT M J. Fast ion transport in solids [M]. Amsterdam: North-Holland, 1979.
[3] NOOKALA M, KUMAR B, RODRIGUES S.  Ionic conductivity and ambient temperature Li electrode reaction in composite polymer electrolytes containing nanosize alumina [J]. J Power Sources, 2002, 111(1): 165-172.
Ionic conductivity and ambient temperature Li electrode reaction in composite polymer electrolytes containing nanosize alumina [J]. J Power Sources, 2002, 111(1): 165-172.
[4] WANG C, XIA Y, KOUMOTO K, SAKAI T.  All solid-state Li/LixMnO2 polymer battery using ceramic modified polymer electrolytes [J]. J Electrochem Soc, 2002, 149(8): A967-A972.
All solid-state Li/LixMnO2 polymer battery using ceramic modified polymer electrolytes [J]. J Electrochem Soc, 2002, 149(8): A967-A972.
[5] PERSI L, CRODE F, SCROSATI B, PLICHTA E, HENDRICKSON M A. Poly(ethylene oxide)-based nanocomposite electrolytes as improved separators for rechargeable lithium polymer batteries: The Li/LiMn3O6 case [J]. J Electrochem Soc, 2002, 149(2): A212-A216.
[6] LI Q, IMANISHI N, TAKEDA Y, HIRANO A, YAMAMOTO O. Four volts class solid lithium polymer batteries with a composite polymer electrolyte [J]. J Power Sources, 2002, 110(1): 38-45.
[7] DIGAR M, HUNG S L, WEN T C. Blending poly(methyl methacrylate) and poly(styrene-co-acrylonitrile) as composite polymer electrolyte [J]. J Appl Polym Sci, 2001, 80(8): 1319-1328.
[8] WIECZOREK W, LIPKA P, ZUKOWSKA G, WYCISLIK H.  Ionic interactions in polymeric electrolytes based on low molecular weight poly(ethylene glycol)s [J]. J Phys Chem, 1998, 102(36): 6968-6974.
Ionic interactions in polymeric electrolytes based on low molecular weight poly(ethylene glycol)s [J]. J Phys Chem, 1998, 102(36): 6968-6974.
[9] BEST A S, ADEBAHR J, JACOBSSON P, MACFARLANE D R. Microscopic interactions in nanocomposite electrolytes [J]. Macromolecules, 2001, 34(13): 4549-4555.
[10] MARCINEK M, BAC A, LIPKA P, ZALEWSKA A, ZUKOWSKA G, BORKOWSKA R, WIECAOREK W. Effect of filler surface group on ionic interactions in PEG-LiClO4-Al2O3 composite polyether electrolytes [J]. J Phys Chem, 2000, 104(47): 11088- 11093.
[11] CHEUNG I W, CHIN K B, GREENE E R, SMART M C, ABBRENT S, GREENBAUM S G, PRAKASH G K S, SURAMPUDI S. Electrochemical and solid state NMR characteriza- tion of composite PEO-based polymer electrolytes [J]. Electrochim Acta, 2003, 48(14/16): 2149-2156.
[12] MEYER W H. Polymer electrolytes for lithium-ion batteries [J]. Adv Mater, 1998, 10(6): 439-448.
[13] SADOWAY D R, HUANG B Y, TRAPA P E, SOO P P, BANNERJEE P, MAYES A M. Self-doped block copolymer electrolytes for solid-state, rechargeable lithium batteries [J]. J Power Sources, 2001, 97/98: 621-623.
[14] SOO P P, HUANG B Y, JANG Y I, CHIANG Y M, SADOWAY D R, MAYES A M. Rubbery block copolymer electrolytes for solid-state rechargeable lithium batteries [J]. J Electrochem Soc, 1999, 146(1): 32-37.
[15] CAPIQLIA C, MUSTARELLI P, QUARTARONE E, TOMASI C, MAQISTRIS A. Effects of nanoscale SiO2 on the thermal and transport properties of solvent-free, poly(ethylene oxide) (PEO)- based polymer electrolytes [J]. Solid State Ionics, 1999, 118: 73-79.
[16] MORITA M, FUJISAKI T, YOSHIMOTO N, ISHIKAWA M. Ionic conductance behavior of polymeric composite solid electrolytes containing lithium aluminate [J]. Electrochim Acta, 2001, 46(10/11): 1565-1569.
[17] SUN H Y, SOHN H J, YAMAMOTO O, TAKEDA Y, IMANISHI N. Enhanced lithium-ion transport in PEO-based composite polymer electrolytes with ferroelectric BaTiO3 [J]. J Electrochem Soc, 1999, 146(5): 1672-1676.
[18] POPALL M, ANDREI M, KAPPEL J, KRON J, OIMA K, OLSOWSKI B. Ormocers as inorganic-organic electrolytes for new solid state lithium batteries and supercapacitors [J]. Electrochim Acta, 1998, 43(10/11): 1155-1161.
[19] KAO H M, CHEN C L. An organic-inorganic hybrid electrolyte derived from self-assembly of a poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymer [J]. Angew Chem, Int Ed, 2004, 43(8): 980-984.
[20] JIANG S, YU D, JI X, AN L, JIANG B. Confined crystallization behavior of PEO in silica networks [J]. Polymer, 2000, 41(6): 2041-2046.
[21] DI NOTO V, ZAGO V, BISCAZZO S, VITTADELLO M. Hybrid inorganic-organic polymer electrolytes: Synthesis, FT-Raman studies and conductivity of {Zr[(CH2CH2O)8.7]ρ/(LiClO4)z}n network complexes [J]. Electrochim Acta, 2003, 48(5): 541-554.
[22] UIBRICHT M, RICHAU K, KAMUSEWITZ H. Chemically and morphologically defined ultrafiltration membrane surfaces prepared by heterogeneous photo-initiated graft polymerization [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1998, 138(2/3): 353-366.
Received date: 2007-12-17; Accepted date: 2008-03-10
Corresponding author: PAN Chun-yue, Professor; Tel: +86-731-8836961; E-mail: panchunyue@sina.com
(Edited by YANG Hua)