文章编号:1004-0609(2016)-12-2580-09
电解液对Ti/RuO2-TiO2电极电容性能的影响
陈志杰1,朱君秋2,邵艳群1,伊昭宇1,唐 电1
(1. 福州大学 材料科学与工程学院,福州 350108;
2. 厦门理工学院 材料科学与工程学院,厦门 361024)
摘 要:采用热分解法在270℃制备钛基RuO2-TiO2氧化物涂层电极材料。利用循环伏安、电化学阻抗谱等方法研究Ti/RuO2-TiO2电极分别在0.5 mol/L H2SO4、0.5 mol/L Na2SO4和 1.0 mol/L NaOH电解液中的超电容行为。结果表明:在酸性溶液和碱性溶液中Ti/RuO2-TiO2电极有较低的电荷转移电阻和优异的赝电容特性,比电容分别达到550 F/g和578 F/g;而在Na2SO4溶液中,该电极的电荷转移电阻较高,表现为典型的双电层电容特征,比电容仅为335 F/g;经历2000次循环充放电测试后,该电极在中性Na2SO4溶液中的稳定性最高,荷电能力仅下降3%;在酸性H2SO4溶液和碱性NaOH溶液中,该电极的荷电能力分别下降17%和29%。结合SEM和能谱分析可知:RuO2-TiO2在Na2SO4溶液中几乎不发生腐蚀,表现出良好的循环稳定性;RuO2-TiO2涂层在NaOH溶液中发生严重的面腐蚀,而在H2SO4溶液中则发生严重的点蚀,导致活性氧化物减少,荷电能力下降。
关键词:超级电容器;氧化物阳极;电解液;反应机理
中图分类号:TM531 文献标志码:A
电化学超级电容器是一种介于蓄电池和传统静电电容器之间的新型储能器件,具有比容量高、功率密度大、充电时间短、循环寿命长等特点。与传统静电电容器相比,超级电容器具有高的能量密度;与蓄电池相比,其具有高的功率密度。由于超级电容器能补充传统电容器及蓄电池的性能间隙,近年来得到国内外学者广泛的关注[1-4]。超级电容器的储能机制包括两类:一类是基于电极表面与溶剂之间界面的双电层电容;另一类是基于电极表面或近表面的赝电容,包括欠电位沉积、电荷镶入及氧化还原反应[5]。不管是双电层电容,还是赝电容,电解液是影响电容性能的重要因素之一。例如,RuO2在酸中的耐腐蚀性较好,因此,含RuO2电极材料常选择硫酸溶液作为电解液[6-8],无定型结构RuO2·nH2O电极在H2SO4溶液中的比电容高达768 F/g[9]。而MnO2在酸中耐腐蚀性较差,不宜使用H2SO4溶液为电解液。如WEN等[10]研究H2SO4和KCl溶液对MnO2涂层电极的影响,认为H+和K+深度均对赝电容有影响,即金属氧化物超级电容器的充电取决于电解液中的阳离子、质子或碱性离子的活动能力。但是在水性溶液中,随着pH值的增大,MnO2涂层电极的比电容在H2SO4溶液中下降速度比KCl溶液快。另外,LIU等[11]研究发现,在H2SO4溶液中加入Fe3+/Fe2+离子能有效提高聚苯胺/SnO2超级电容器的赝电容行为,当Fe3+/Fe2+浓度为0.4 mol/L 时,超级电容器的性能最好,其在1 A/g电流密度下的比容量达到 1172 F/g。MAYRAND-PROVENCHER等[12]研究认为具有高的导电性和质子交换的质子型离子溶液可提高金属氧化物电极电容器的比电容。由此可见,电解液对超级电容器的电容性能的影响极大,不同的电极材料体系应选用不同的电解体系。
RuO2由于导电性好、电化学性能稳定、比电容量高,被认为是最优秀的超级电容器电极材料之一[13-15]。有关RuO2及其复合氧化物电极材料的制备及电化学能研究的文献报道很多[3, 16],但系统研究电解液对RuO2及其复合氧化物电极材料电容性能影响的报道较少。鉴于此,本文作者以0.5 mol/L H2SO4、0.5 mol/L Na2SO4和1.0 mol/L NaOH溶液为电解液,系统研究RuO2-TiO2复合氧化物电极材料分别在酸性、中性和碱性溶液中的储能方式、功率特性以及循环充放电稳定性等电容行为。研究结果可作为含RuO2电极材料在选择电解液时的重要参考依据。
1 实验
1.1 氧化物电极材料的制备
钛基体预处理步骤:选用喷砂过的工业纯钛TA2经除油、清洗干净后在浓度为10%(质量分数)的草酸溶液中刻蚀1.5 h, 使基体呈现均匀的麻面,以增加表面粗糙度和涂层与基体的结合力。
RuO2-TiO2氧化物涂层的制备:以乙醇为溶剂,将含Ru质量分数为37%的RuCl3·xH2O和质量分数为15%的三氯化钛溶液配制成前驱体涂液(金属R和Ti的摩尔比为40:60)。用软毛笔将前躯体涂液涂覆于经预处理的钛基体表面,然后在红外灯下烘干,烘干后放入马弗炉中进行热氧化12 min,氧化温度为270 ℃,出炉后空冷。重复上述步骤,直到涂液用尽。RuO2的载量为1.0 mg/cm2。
1.2 电化学测试
电化学测试设备为瑞士万通Autolab PGST-302N电化学工作站和上海辰华CHI660D电化学工作站。测试系统为三电极体系,以Ag/AgCl 电极为参比电极,辅助电极为Ti电极,用热分解法制备的RuO2-TiO2氧化物电极为工作电极,面积为1 cm2。电解液分别为0.5 mol/L 的H2SO4溶液、0.5 mol/L 的Na2SO4溶液和1 mol/L的NaOH溶液。电化学阻抗谱和恒流充放电在AUTOLAB 进行,交流阻抗的测试电位为0 V (Na2SO4溶液)、-0.45 V(NaOH溶液)、0.45 V(H2SO4溶液),振幅 10 mV,测试频率范围5 mHz~10 kHz;恒流充放电测试电位窗口为-0.5~0.5 V(Na2SO4溶液)、-0.9~0 V(NaOH溶液)、0~0.9 V(H2SO4溶液),电流密度5 mA/cm2。循环寿命测试在上海辰华CHI660D 电化学工作站上进行,测试电位窗口为-0.5~0.5 V (Na2SO4溶液)、-0.9~0 V(NaOH溶液)、0~0.9 V(H2SO4溶液),扫描速度100 mV/s。
1.3 物相及形貌分析
X射线衍射(XRD)分析在日本理学Rigaku,Ultimate III衍射仪上进行,Cu靶(Kα),电压40 kV,电流40 mA。采用德国ZEISS SUPRA55扫描电镜观察循环充放电寿命测试前后的阳极形貌,采用Oxford的X-Max能谱仪进行表面成分分析。
2 结果与讨论
2.1 循环伏安特性分析
图1所示为Ti/RuO2-TiO2电极分别在0.5 mol/L H2SO4、0.5 mol/L Na2SO4、1 mol/L NaOH为电解液的循环伏安曲线,扫描速度为5 mV/s。
由图1可看出,在3种不同电解液中电极的电位窗口各不相同,在H2SO4溶液的电位窗口为0~0.9 V(氧化还原峰在0.5 V附近),在Na2SO4溶液的电位窗口为-0.5~0.5 V(无氧化还原峰),在NaOH溶液的电位窗口为-0.9~0 V(氧化还原峰在-0.4 V附近)。电位窗口(或氧化还原峰)随电解液的改变而不同,这与Ru阳极的反应机理发生变化以及溶液pH值有关。RuO2的氧化还原反应电容的增加主要是通过表面氧化膜的活性点与质子之间的交换反应实现。RuO2涂层电极的质子反应机理[17-18]可表示为
RuO2+H++e  RuOOH (1)
RuOOH (1)
式(1)可简化为
Ru4++e  Ru3+ (2)
Ru3+ (2)
根据Nernst方程[19],式(1)的阳极氧化电位可表示为
 (3)
(3)
式(3)可简化为
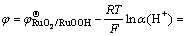
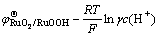 (4)
(4)
式中: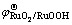 为标准电极电位;R为摩尔气体常数(8.3143 J/(K·mol));T为绝对温度(301 K);F为法拉第常数(96485.3 C/mol);
为标准电极电位;R为摩尔气体常数(8.3143 J/(K·mol));T为绝对温度(301 K);F为法拉第常数(96485.3 C/mol);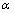 为活度(
为活度(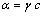 );
);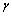 为活度因子;c为溶液浓度。即电极的氧化还原反应电位与pH值呈线性关系,随pH值的增大,氧化还原电位逐渐减小。
为活度因子;c为溶液浓度。即电极的氧化还原反应电位与pH值呈线性关系,随pH值的增大,氧化还原电位逐渐减小。
由图1还可以看出,在酸性和碱性电解液中,电极的循环伏安面积相差不大,且均具有明显的氧化还原峰,说明在酸性和碱性溶液中,电极具有较强的电荷存储能力和良好的赝电容性能。而在Na2SO4溶液中,电极的伏安曲线保持理想电容的矩形特征,没有出现氧化还原峰,说明主要以双电层电容为储能方式。不同溶液中的电容表现同样可由方程(1)和方程(5)来解释说明。RuO2的反应机制是通过H+作为(在O2-、OH-)中间介质的扩散反应来实现的:
O2++H+  OH- (5)
OH- (5)
在正电荷浓度相同的0.5 mol/L Na2SO4和0.5 mol/L H2SO4溶液中,由于H2SO4溶液的H+浓度远大于Na2SO4溶液的,因此,在H2SO4溶液中,除了双电层方式储能外,还具有很强烈的法拉第赝电容反应,因而其电容明显大于Na2SO4溶液(Na2SO4溶液为中性,其H+和OH-浓度相等,浓度在1×10-7 mol/L,因此,不能为RuO2的赝电容反应提供足够量的质子)。然而,NaOH溶液中的H+浓度明显比Na2SO4的少,但其比电容却明显比Na2SO4的大。这主要是由于Ru在碱性溶液中具有更高的氧化电位,可从Ru2+到Ru7+,而在酸性溶液中从Ru2+到Ru6+,这也是在低扫速下,NaOH溶液的比电容甚至比H2SO4溶液略大的原因。当Ru的价态超过+6时,电极电位也会随之提高。在这种情况下,由于需要增加电荷变化来平衡Ru离子的价态变化,这会促进形成一种伴有(O2-、OH-)相变的协调机制,式(5)持续向左反应,为赝电容反应提供质子。所以在碱性溶液中,RuO2同样能表现出优异的赝电容特性。
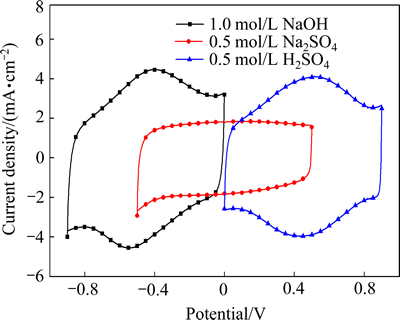
图1 扫描速度为5 mV/s时Ti/RuO2-TiO2电极在不同电解液的循环伏安曲线图
Fig. 1 CV curves of Ti/RuO2-TiO2 electrode in different electrolytes at scan rate of 5 mV/s
2.2 功率特性分析
比能量密度、比功率密度是储能设备两个非常重要的性能指标。能量密度和功率密度之间的关系在实际应用和理论上都非常重要[20]。通过下列方程可计算电极的能量密度(E)和功率密度(P):
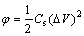 (6)
(6)
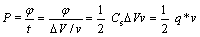 (7)
(7)
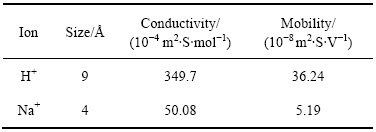
式中:Cs为比电容;△V为循环伏安电位窗口;q*为伏安积分电量;v为扫描速度。根据方程(6)和(7)计算的能量密度和功率密度结果取对数作图,该曲线称为Ragone曲线。如图2所示,电极在3种电解液下的比能量均随比功率的增加而减小。其中,相同功率密度下,在Na2SO4溶液中获得能量密度均小于另外两种电解液,这与在Na2SO4电解液中氧化钌的赝电容活性未能得到很好的发挥有关。对比在NaOH和H2SO4电解液中获得Ragone曲线发现,在低功率密度区间(<5418 W/kg),电极在NaOH电解液中能获得更高的能量密度;当功率密度高于5418 W/kg时,电极在硫酸溶液中能获得更高的能量密度。这一现象与两种电解液中阳离子的半径以及离子导电能力有关。如表1所示,在水性溶液中Na+的半径(等于4 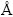 )小于H+半径(等于9
)小于H+半径(等于9 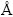 ),在较小的功率密度下充放电时,离子有充足的时间迁移至涂层的近表面和内部,因此,能形成更高双电层电容。离子半径越小,越能往涂层内部迁移,即涂层内活性点也能得到利用,因此,可获得更高的能量密度;随之功率密度提高,即充放电的速度加快时,电荷存储主要发生电极表面,此时离子的导电能力和在电解液中的迁移能力成为决定电极材料比能量的关键。从表1可以看出,H+离子的导电率和迁移能力均远高于Na+离子的,因此,高功率密度下,电极在硫酸电解液中可以获得更高的能力密度。
),在较小的功率密度下充放电时,离子有充足的时间迁移至涂层的近表面和内部,因此,能形成更高双电层电容。离子半径越小,越能往涂层内部迁移,即涂层内活性点也能得到利用,因此,可获得更高的能量密度;随之功率密度提高,即充放电的速度加快时,电荷存储主要发生电极表面,此时离子的导电能力和在电解液中的迁移能力成为决定电极材料比能量的关键。从表1可以看出,H+离子的导电率和迁移能力均远高于Na+离子的,因此,高功率密度下,电极在硫酸电解液中可以获得更高的能力密度。
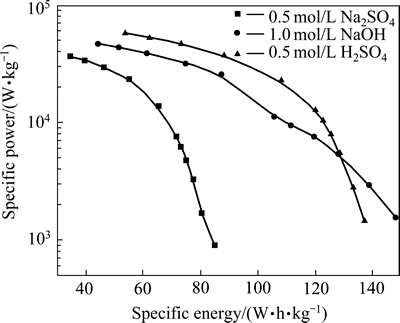
图2 不同电解液测得的Ti/RuO2-TiO2电极的比功率与比能量之间的关系
Fig. 2 Relationship between specific power and specific energy of Ti/RuO2-TiO2 electrode measured in different electrolytes
表1 H+和Na+在无限稀释水溶液中的离子半径、电导率和迁移率[10]
Table 1 Ionic radii, conductivity and mobilities of H+ and Na+ in infinite diluted aqueous solutions[10]
2.3 交流阻抗谱分析
图3所示为Ti/RuO2-TiO2电极在不同电解液中测得的Nuquist图全谱图和等效电路图。其中图3(a)所示为Nuquist全谱图;图3(b)所示为等效电路图;图3(c)为中高频区放大图。根据等效电路拟合数据在表2中给出。在高频区,阻抗曲线与实轴的截距所对应的值,即为等效串联电阻值(称为Rs),包含电极内阻,电解液电阻和活性材料与集流体及电解液之间的接触电阻。表2中所列的3组电极的Rs值基本相同。中高频区半圆源于双电层形Cdl成过程中固体氧化物(电子电导率)与液体电解液相(离子电导率)之间不同的电导率引起的电极电解液界面不连续的电荷转移过程产生的,称为电荷转移电阻(Rct),可通过与高频区形成的半圆直径计算得出,在硫酸溶液中Rct有最小值(见表2)。在Na2SO4电解液中Rct有最大值,约为另外两组数据的两倍。Rct与交换电流密度成反比关系(Rct=(RT)/ (nFJ0)),即Rct越小,交换电流密度J0越大。交换电流密度可以用来描述一个电极反应得失电子的能力,反映一个电极反应进行的难易程度。由此可以看出,在Na2SO4电解液中电极的氧化还原反应最难进行,因此,循环伏安表现为典型的双电层电容特征。
低频区有一近似平行于Zim(虚轴)的直线,这是非理想可极化电极的特征,这与可逆氧化还原反应赝电容CF形成过程有关。偏离虚轴的程度与扩散阻抗Zw有关。Zw反映了电解液中电极材料发生氧化还原的离子的扩散情况,直观表现为曲线的斜率,斜率越大,性能越好。从表2中可以看出,以硫酸为电解液的法拉第赝电容CF都具有最大值。远高于以Na2SO4为电解液获得数据,但与以NaOH为电解液获得拟合数据接近。扩散阻抗Zw大小顺序刚好与赝电容CF大小顺序一致,说明离子在电极材料中的扩散能力对电极材料的储能能力有重要的影响。
2.4 恒流充放电分析
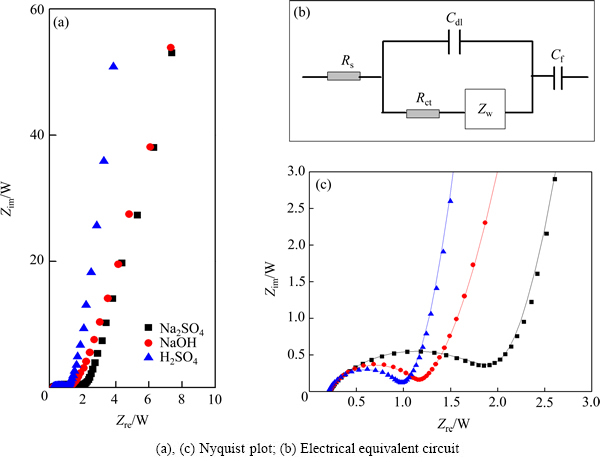
图3 Ti/RuO2-TiO2电极分别在不同溶液中获得的Nyquist图
Fig. 3 Nyquist plots and electrical equivalent circuit of Ti/RuO2-TiO2 electrodes measured in different electrolytes
表2 Ti/RuO2-TiO2电极在3种电解液中得到的交流阻抗拟合参数
Table 2 Electrochemical parameters of Ti/RuO2-TiO2 electrodes as calculated from EIS data
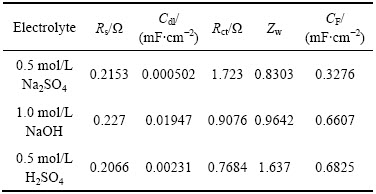
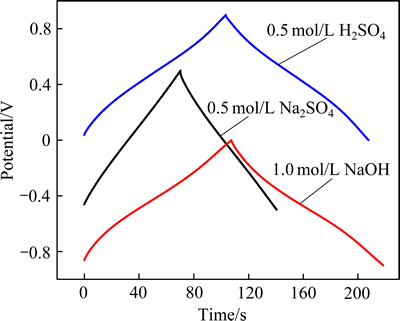
图4 电流密度为5 mA/cm2时电极在不同溶液中测试的恒流充放电曲线
Fig. 4 Constant current charge-discharge curves of Ti/RuO2- TiO2 electrode in different electrolytes at current density of 5 mA/cm2
图4所示为Ti/RuO2-TiO2电极在不同电解液下的恒流充放电曲线。由图4可看出,在3种电解液下,充放电曲线均具有较好的对称性,说明三者均具有较好的可逆性。但是与以Na2SO4溶液为电解液不同,以H2SO4溶液和NaOH溶液为电解液的充电曲线和放电的电压-时间曲线不是成线性关系的,原因是电极在H2SO4溶液和NaOH溶液的氧化还原反应延缓电极电位的升高或下降,这说明电极在H2SO4溶液和NaOH溶液中具有典型的赝电容特性,与循环伏安曲线的结果一致。根据放电曲线可计算出电极的比电容Cs,它与放电时间成正比,计算公式为:Cs=It/(Δφm)(式中I为放电电流密度,A;t是放电时间,s;Δφ是电位差值,V;m是单位面积Ti基体所含有的RuO2的质量,g),计算结果如表3所示。在H2SO4溶液和NaOH溶液的比电容相近,都远高于以Na2SO4溶液为电解的比电容。
表3 电流密度为5 mA/cm2时电极在不同电解质溶液的比电容
Table 3 Specific capacitance (Cs) of Ti/RuO2-TiO2 electrode calculated from discharge curves measured in different electrolytes at current density of 5 mA/cm2

2.5 循环充放电稳定性分析
电极的循环充放电稳定性是考察电容器是否满足工业应用的重要指标。图5所示为Ti/RuO2-TiO2电极在第一圈和第2000次循环的伏安曲线。图5(a)所示为以Na2SO4溶液为电解液测得的伏安曲线。从图5(a)中可以看出,在中性溶液中测试2000圈后,其循环伏安曲线基本不变,说明电极在Na2SO4溶液中较为稳定。而在H2SO4溶液和NaOH溶液中测试2000个循环后(扫速为100 mV/s),其曲线面积明显缩小。通过积分电量计算,经历2000次循环充放电测试后,电极在中性Na2SO4溶液中有最高的稳定性,荷电能力仅下降3%;酸性H2SO4溶液和碱性NaOH溶液中,电极的荷电能力分别下降了17%和29%。
2.6 微观形貌分析
图6所示为Ti/RuO2-TiO2电极在不同溶液中测试循环寿命前后的表面形貌SEM像。图6(a)所示为新鲜样品的表面形貌图,呈现典型的“泥裂”形貌。图6(b)所示为在Na2SO4电解液中测试2000次循环充放电后氧化物涂层的形貌,与图6(a)相比,几乎没有区别,说明在RuO2-TiO2复合涂层在Na2SO4电解液未发生明显的溶解现象。图6(c)所示为在NaOH电解液中测试2000次循环充放电后氧化物涂层的形貌,与图6(a)相比,RuO2-TiO2涂层变得更加平滑,尤其是裂纹边界变得非常的圆滑,说明RuO2-TiO2涂层在NaOH溶液中呈现均匀溶解的特点,即在循环充放电过程存在严重的面腐蚀。图6(d)所示为在H2SO4电解液中测试2000次循环充放电后氧化物涂层的形貌。由图6(d)可以看出,在氧化物涂层表面出现许多点蚀坑,即在循环充放电过程存在严重的点蚀。
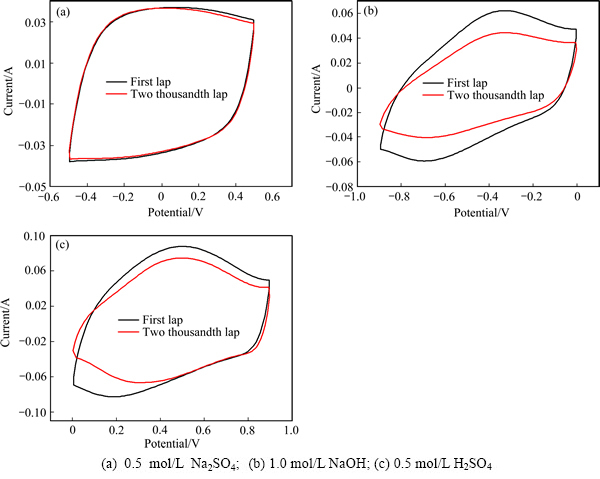
图5 扫描速度100 mV/s时Ti/RuO2-TiO2电极在不同电解液中的第一圈和第2000圈的伏安曲线
Fig. 5 Charge-discharge cycling test in different electrolytes by CV cycling at scan rate of 100 mV/s
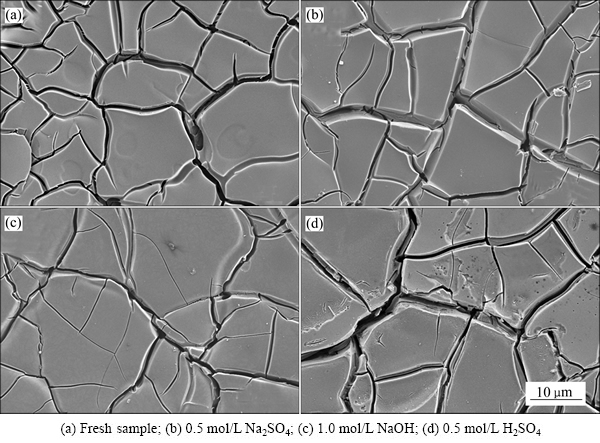
图6 Ti/RuO2-TiO2电极在不同电解液进行循环寿命测试前后的表面形貌
Fig. 6 Surface morphologies of Ti/RuO2-TiO2 electrodes before and after charge-discharge cycling at different electrodes
为进步一步确认各电极在循环充放电测试后的腐蚀情况,对各氧化物涂层进行能谱分析,结果列于表4中。由表4可以看出,新鲜的样品表面Ru和Ti的摩尔比要低于前驱体溶液中Ru和Ti的摩尔比,说明Ru有向涂层内部扩散的趋势,使得表面钌原子浓度低于涂层内部。在硫酸钠电解液中循环充放电测试后,电极表面钌原子浓度略高于新鲜样品,但区别非常的小,说明RuO2-TiO2涂层在硫酸钠溶液仅发生非常轻微的腐蚀,这与SEM像变化结果一致。在NaOH溶液中测试循环充放电测试后,涂层表面钌原子的浓度要明显高于新鲜样品,说明RuO2-TiO2氧化物涂层在NaOH发生较为严重的溶解,使得具有更高钌原子浓度的内层暴露在外。同样可以看到,在H2SO4溶液中测试万循环充放电后,氧化物涂层表面的钌原子浓度达到最高值,结合SEM像可知,氧化物涂层在硫酸溶液中发生严重的点蚀,使得内层高更高钌原子浓度的涂层暴露出来。
表4 循环寿命测试前后电极氧化物涂层表面Ru和Ti的摩尔比
Table 4 Mole ratio of Ru and Ti before and after charge- discharge cycling in different electrolytes

2.7 涂层的物相分析
为进一步分析3种电解液对循环充放电测试后涂层的物相是否造成影响。对循环充放电测试前后的样品进行物相分析,以研究电解液对氧化物涂层物相是否造成重要的影响。图7所示为Ti/RuO2-TiO2电极在3种电解液中测试循环寿命前后的XRD谱。由图7可见,在循环寿命测试前以及测试后的XRD谱中仅存在基体钛的晶化衍射峰,未出现氧化物的晶化衍射峰。但在20°~38°均出现明显的非晶包,说明RuO2-TiO2复合氧化物以非晶态结构形式存在。在Na2SO4电解液中测试后的样品的XRD谱线与测试前样品的XRD谱线接近,无明显变化。而在NaOH溶液和H2SO4溶液中测试后的样品的XRD谱线在非晶包变得平缓,尤其是以NaOH为电解液测试后的样品其非晶包最为平缓,说明表面涂层在NaOH溶液和H2SO4溶液中有一定的溶解,且在NaOH溶液中溶解较为严重。这一结果同样证实电极荷电能力的衰退与氧化物涂层的溶解密切相关。
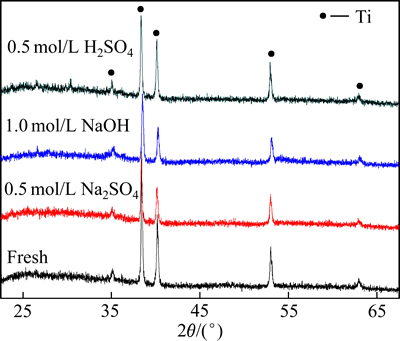
图7 Ti/RuO2-TiO2电极在不同电解液下测试循环寿命前后的XRD谱
Fig. 7 XRD patterns of Ti/RuO2-TiO2 samples before and after charge-discharge cycling test in different electrolytes
3 结论
1) 电解液对电极材料充放电过程中的电荷转移电阻、电极材料的氧化还原能力以及材料的腐蚀有重要的影响。
2) 酸性硫酸电解液和碱性氢氧化钠电解液都有利RuO2赝电容性能的发挥,因此,可获得较高的比电容,中性硫酸钠电解液则不利于RuO2赝电容性能的发挥,其比电容值较低。
3) 在2000次循环寿命测试过程,RuO2-TiO2氧化物涂层在酸性硫酸电解液和碱性氢氧化钠电解液分别发生严重的点蚀和面蚀,导致2000次循环充放电后荷电能力分别下降了17%和29%;而在硫酸钠溶液不易发生溶解,由此表现出良好的稳定性,荷电能力仅下降3%。
REFERENCES
[1] LI Xue-liang, XING Yan, WANG Hua, WANG Hua-lin, WANG Wei-dong, CHEN Xiang-ying. Synthesis and characterization of uniform nanoparticles of gamma-Mo2N for supercapacitors[J]. Transactions of Nonferrous Metals Society of China, 2009, 19(3): 620-625.
[2] MILLER J R, SIMON P. Electrochemical capacitors for energy management[J]. Science, 2008, 321(5889): 651-652.
[3] WANG G, ZHANG L, ZHANG J. A review of electrode materials for electrochemical supercapacitors [J]. Chemical Society Reviews, 2012, 41(2): 797-828.
[4] 朱君秋, 王 欣, 娄长影, 邵艳群, 张 腾, 唐 电. 烧结温度对热分解制备的RuO2电容性能的影响[J]. 金属热处理, 2013, 38(8): 65-68.
ZHU Jun-qiu, WANG Xin, LOU Chang-ying, SHAO Yan-qun, ZHANG Teng, TANG Dian. Influence of sintering temperature on capacitive properties of RuO2 electrodes prepared by thermal decomposition[J]. Heat Treatment of Metals, 2013, 38(8): 65-68.
[5] ZHENG J P, HUANG C K. Electrochemical behavior of amorphous and crystalline ruthenium oxide electrodes[J]. Journal of New Materials for Electrochemical Systems, 2002, 5(1): 41-46.
[6] 邵艳群, 伊昭宇, 朱君秋, 娄长影, 马晓磊, 唐 电. 高比能量Ti/(Ir0.3Sn(0.7-x)Cex)O2电极材料的制备及其电化学性能[J]. 中国有色金属学报, 2015, 25(3): 714-719.
SHAO Yan-qun, YI Zhao-yu, ZHU Jun-qiu, LOU Chang-ying, MA Xiao-lei, TANG Dian. Preparation and electrochemical performance of Ti/(Ir0.3Sn(0.7-x)Cex)O2 electrode materials with high specific energy[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(3): 714-719.
[7] 朱君秋, 路恒达, 王 欣, 张 腾, 邵艳群, 唐 电. 成分配比对RuO2·Ta2O5二元氧化物电容性能的影响[J]. 稀有金属材料与工程, 2013, 42(3): 554-559.
ZHU Jun-qiu, LU Heng-da, WANG Xin, ZHANG Teng, SHAO Yan-qun, TANG Dian. Effect of composition on the structure and capacitive performance of Ti/RuO2-Ta2O5 electrodes[J]. Rare Metal Materials and Engineering, 2013, 42(3): 554-559.
[8] 孙俊梅, 王 欣, 魏宗平, 邵艳群, 张 腾, 唐 电. 烧结温度对Ti/RuO2-CeO2超电容性能的影响[J]. 中国稀土学报, 2011, 29(6): 718-723.
SUN Jun-mei, WEN Xin, WEI Zong-ping, SHAO Yan-qun, ZHANG Teng, TANG Dian. Effect of sintering temperature on the capacitive performance of Ti/RuO2-CeO2 electrodes[J]. Journal of the Chinese Society of Rare Earths, 2011, 29(6): 718-723.
[9] SHAO Y Q, YI Z Y, HE C, ZHU J Q, TANG D. Effects of annealing temperature on the structure and capacitive performance of nanoscale Ti/IrO2-ZrO2 electrodes[J]. Journal of the American Ceramic Society, 2015, 98(5): 1485-1492.
[10] WEN S, LEE J W, YEO I H, PARK J, MHO S. The role of cations of the electrolyte for the pseudocapacitive behavior of metal oxide electrodes, MnO2 and RuO2[J]. Electrochimica Acta, 2004, 50(2/3): 849-855.
[11] LIU Tian-tian, ZHU Yin-hai, LIU En-hui, LUO Zhen-yu, HU Tian-tian, LI Zeng-peng, DING Rui. Fe3+/Fe2+ redox electrolyte for high-performance polyaniline/SnO2 supercapacitors[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(8): 2661-2665.
[12] MAYRAND-PROVENCHER L, ROCHEFORT D. Influence of the conductivity and viscosity of protic ionic liquids electrolytes on the pseudocapacitance of RuO2 electrodes[J]. Journal of Physical Chemistry C, 2009, 113(4): 1632-1639.
[13] HU C C, CHANG K H, LIN M C, WU Y T. Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation supercapacitors[J]. Nano Letters, 2006, 6(12): 2690-2695.
[14] GALIZZIOLI D, TANTARDINIT F, TRASATTI S. Ruthenium dioxide: A new electrode material [J]. Journal of Applied Electrochemistry, 1975, 5: 203-214.
[15] 刘 泓, 甘卫平, 郭桂全, 刘继宇, 李 祥, 郑 峰. RuO2·nH2O薄膜的制备以及物相演变和伏安特性[J]. 中国有色金属学报, 2010, 20(3): 521-528.
LIU Hong, GAN Wei-ping, GUO Gui-quan, LIU Ji-yu, LI Xiang, ZHENG Feng. Preparation, phase transition and cyclic voltammetric properties of RuO2·nH2O film[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(3): 521-528.
[16] 伊昭宇, 贺 冲, 朱君秋, 邵艳群, 唐 电. Ti/Ru0.4Sn(0.6-x)MnxO2氧化物电极电容性能研究[J]. 中国有色金属学报, 2016, 26(4): 821-828.
YI Zhao-yu, HE Chong, ZHU Jun-qiu, SHAO Yan-qun, TANG Dian. Capactive properties of Ti/Ru0.4Sn(0.6-x)MnxO2 electrodes[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(4): 821-828.
[17] POCRIFKA L A, GON ALVES C, GROSSI P, COLPA P C, PEREIRA E C. Development of RuO2-TiO2 (70-30)mol% for pH measurements[J]. Sensors and Actuators B: Chemical, 2006, 113(2): 1012-1016.
[18]  B, KALINAUSKAS P, JUODKAZIS K. Light energy accumulation using Ti/RuO2 electrode as capacitor[J]. Journal of Solid State Electrochemistry, 2009, 14(5): 741-746.
B, KALINAUSKAS P, JUODKAZIS K. Light energy accumulation using Ti/RuO2 electrode as capacitor[J]. Journal of Solid State Electrochemistry, 2009, 14(5): 741-746.
[19] CHEN X, CHEN K, WANG H, XUE D. A colloidal pseudocapacitor: Direct use of Fe(NO3)3 in electrode can lead to a high performance alkaline supercapacitor system[J]. Journal of Colloid and Interface Science, 2015, 444: 49-57.
[20] PELL W, CONWAY B. Quantitative modeling of factors determining Ragone plots for batteries and electrochemical capacitors[J]. Journal of Power Sources, 1996, 63(2): 255-266.
Effect of electrolyte on capacitive performance of Ti/RuO2-TiO2 electrodes
CHEN Zhi-jie1, ZHU Jun-qiu2, SHAO Yan-qun1, YI Zhao-yu1, TANG Dian1
(1. College of Materials Science and Engineering, Fuzhou University, Fuzhou 350108, China;
2. College of Materials Science and Engineering, Xiamen University of Technology, Xiamen 361024, China)
Abstract: RuO2-TiO2 coating were prepared by thermal decomposition method on pure titanium TA2 substrate at 270 ℃. The supercapacitor behavior of RuO2-TiO2 coating was investigated by cyclic voltammetry(CV), electrochemical impedance spectroscopy (EIS) and charging-discharging test in 0.5 mol/L H2SO4, 0.5 mol/L Na2SO4 and 1.0 mol/L NaOH electrolytes, respectively. The results show that the Ti/RuO2-TiO2 electrode has low charge transfer resistance in acidic and alkaline electrolytes, and the specific capacitance of 550 F/g and 578 F/g are obtained in these two electrolytes, respectively. Conversely, the electrode has relative higher charge transfer resistance and lower specific capacitance (335 F/g) in neutral Na2SO4 electrolyte than that of in acidic and alkaline electrolytes. However, the electrode losing 17% and 29% of the maximum capacity after 2000 charging-discharging cycles in acidic and alkaline electrolytes, respectively, but just only 3% lost in neutral Na2SO4 solution. Combined with the SEM and EDS analysis, the electrode is hardly corroded in Na2SO4 solution, thus exhibits good cycle stability. In contrast, Ti/RuO2-TiO2 electrodes occur serious surface corrosion in NaOH solution, while occurring serious pitting corrosion in H2SO4 solution. Severe corrosion results in a significantly reduction of active substance, and thus reduces the charge capacity of the electrode.
Key words: supercapacitor; oxide anode; electrolyte; reaction mechanism
Foundation item: Project(11374053) supported by the National Natural Science Foundation of China; Project (2015J01190) supported by the Natural Science Foundation of Fujian Province, China
Received date: 2015-11-06; Accepted date: 2016-07-25
Corresponding author: SHAO Yan-qun; Tel: +86-591-22866532; E-mail: yqshao1989@163.com
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(11374053);福建省自然科学基金资助项目(2015J01190)
收稿日期:2015-11-06;修订日期:2016-07-25
通信作者:邵艳群,副教授,博士,电话:0591-22866532;E-mail: yqshao1989@163.com