J. Cent. South Univ. Technol. (2008) 15: 593-598
DOI: 10.1007/s11771-008-0111-y
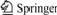
Electro-oxidation treatment of Sn/PANI electrode and
electrocatalytic activity of Pt/Sn hydroxide/PANI composite electrodes
ZHOU Hai-hui(周海晖), PENG Zheng(彭 铮), JIAO Yong-gang(焦勇刚),
LIAO Jie(廖 杰), KUANG Ya-fei(旷亚非)
(College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China)
Abstract: After being electro-oxidized by cyclic voltammetry(CV) method in 0.5 mol/L H2SO4 solution or in 0.2 mol/L H2O2+0.5 mol/L H2SO4 solution, the Sn/polyaniline (PANI) electrodes were modified with Pt microparticles by pulse galvanostatic method, thus Pt/Sn hydroxide/PANI electrodes were prepared. The electrocatalytic activities of the Pt/Sn/PANI electrode and Pt/Sn hydroxide/PANI electrode for formaldehyde electro-oxidation were investigated by CV method. The effects of deposition charges (Qdep) of PANI, Sn and Pt, scan rate and formaldehyde concentration on the electrocatalytic activity of Pt/Sn hydroxide/PANI electrode were also studied. The results show that the electrocatalytic activities of the Pt/Sn hydroxide/PANI electrodes are much higher than those of the Pt/Sn/PANI electrode.
Key words: Sn; Sn hydroxide; formaldehyde; polyaniline; electrocatalytic activity; electro-oxidation
1 Introduction
The electro-oxidation of small organic molecules (such as formaldehyde, methanol, ethanol and so on) has been studied in the last 20 years mainly because these compounds can be applied as fuel of the anodic reaction in fuel cells[1], which have various advantages such as high energy density, portability and pollution-free speciality and so on[2-3]. At present, the main problem to be faced with in this field is how to prepare an efficient catalyst with good activity, economic cost and good stability. Platinum (Pt)-based electrodes are considered the most important electrocatalytic materials. However, the high price of Pt and the strong adsorption of intermediate species (such as CO) that reduce the catalytic activity and stability of Pt forbid the Pt-based electrodes’ practical use[4]. In order to improve the catalytic efficiency of Pt catalyst and inhibit the formation of adsorbed CO, Pt has been alloyed with secondary metals. Pt-Ru and Pt-Sn alloys have been widely investigated and led to good results[5-6]. However, the price of Ru is also high; the catalytic activity of Pt-Sn alloy depends on the preparation method, and the performance of Pt-Sn alloy decreases at high anodic current densities because the adsorption and dehydro- genation of methanol on Pt-Sn alloy become more difficult than those on Pt[6]. Furthermore, the long-term stability of Sn in acidic solution may be a problem.
Recently, some researches have shown that dispersion of Pt particles into or onto metal oxide layers can promote the catalytic performance of Pt for small organic molecules oxidation. Several kinds of metal oxides such as WO3[7], Sn oxide[8-9], Ru oxide[10] and IrO2[11] show good assistant effect with Pt for the oxidation of methanol. However, all these Pt-modified metal oxide electrodes were prepared by thermal method or sol-gel method. Electrochemical method is a kind of economical and convenient method, which has potential application in industrial production. In our previous work, Pt nanoparticles were dispersed on nanofibrous polyaniline(PANI) electro-synthesized by pulse galvanostatic method(PGM) and Pt/PANI electrode was prepared. The research result shows that the catalytic activity of this Pt/PANI electrode for methanol oxidation is higher than that of pure Pt[12]. In this work, high-dispersed Sn particles were deposited onto the nanofibrous PANI surface by PGM to prepare Sn/PANI electrodes, then the Sn particles were anodic-oxidized by electrochemical method and Sn hydroxide/PANI electrodes were prepared. After Pt particles were modified onto the Sn hydroxide/PANI electrodes by PGM, the Pt/Sn hydroxide/PANI electrodes were prepared and their electrocatalytic activities for electro-oxidation of formaldehyde were studied.
2 Experimental
2.1 Reagent and apparatus
Aniline was purified by repeated distillation and stored under nitrogen gas. All other reagents were of analytical grade. All solutions were prepared with twice distilled water. The working electrode was a stainless steel disc with a surface area (S) of 0.25 cm2. A platinum foil was used as counter electrode. The electrosynthesis and electrodeposition processes were carried out by the pulse galvanostatic system (Handan Instrument Factory, China) using a two-electrode cell. The cyclic voltammetry(CV) experiments were performed by a CHI660B electrochemical workstation (Shanghai Chenhua Instrument Factory, China) in a three-electrode cell, using a saturated calomel electrode (SCE) as reference electrode at room temperature.
2.2 Preparation of Sn/PANI electrodes and their electro-oxidation treatments
Nanofibrous PANI was synthesized on stainless steel(SS) by PGM from 0.3 mol/L aniline+1 mol/L HNO3 solution (temperature, 15 ℃; anodic current density, 1.2 mA/cm2; ton?toff (the ratio of “on” pulse period to “off” pulse period), 90 ms?10 ms)[13]. Then Sn was electrodeposited on the PANI electrodes by PGM from 0.05 mol/L SnCl2+0.5 mol/L H2SO4 solution (temperature, 30 ℃; cathodic current density, 1.5 mA/cm2; ton ? toff, 0.1 ms ? 9.9 ms). After the Sn/PANI electrodes were prepared, they were electro-oxidized in 0.5 mol/L H2SO4 or 0.2 mol/L H2O2+0.5 mol/L H2SO4 solution using the CV method (scan potential range, from -0.5 to 0.9 V; scan rate, 50 mV/s). Thus, the Sn hydroxide/PANI electrodes were prepared. Then the surface Laman spectra of the Pt/Sn hydroxide/PANI electrodes were recorded using a Labram-010 triple monochromator spectrometer (Jobin Yvon Company, France). An argon ion laser with wavelength of 514. 5 nm was used as the excitation source.
2.3 Preparation of Pt/Sn hydroxide/PANI electrodes and their electrocatalytic activities for formaldehyde oxidation
Pt was electrodeposited on the Sn/PANI electrode and the Sn hydroxide/PANI electrodes by PGM from 7 mmol/L H2PtCl6+0.5 mol/L H2SO4 solution (temperature, 30 ℃; cathodic current density, 1.2 mA/cm2; ton ?toff, 0.1 ms?9.9 ms), Pt/Sn/PANI electrode and Pt/Sn hydroxide/ PANI electrodes were thus prepared. The electrode electro-oxidized in 0.2 mol/L H2O2+0.5 mol/L H2SO4 was registered as Pt/Sn hydroxide/PANI electrode Ⅰ and the electrode electro-oxidized in 0.5 mol/L H2SO4 was registered as Pt/Sn hydroxide/PANI electrode Ⅱ.
Then the electrocatalytic activities of these three kinds of electrodes for formaldehyde electro-oxidation were examined by CV method in 0.8 mol/L HCHO+0.5 mol/L H2SO4 solution (scan potential range, 0-1.1 V; scan rate, 50 mV/s).
3 Results and discussion
3.1 CV electro-oxidation treatment of Sn/PANI elec- trode and surface Raman spectrum of electrode after electro-oxidation
Fig.1 shows the cyclic voltammograms of the Sn/ PANI electrode in 0.5 mol/L H2SO4 and in 0.2 mol/L H2O2+0.5 mol/L H2SO4, respectively. It can be seen from Fig.1 that there are two anodic oxidation peaks in both curves (a) and (b). Although an oxidation peak of PANI electrode also appears in 0.5 mol/L H2SO4 solution around the potential of 0.2 V, the peak current density is only 2-8 mA/cm2[13], which is considerably lower than that of the second anodic oxidation peak in Fig.1 (116.75 mA/cm2). This indicates that the second anodic oxidation peak is mainly caused by the further oxidation of Sn. When comparing the two CV curves, it can be found that in curve (a), Ep1=-0.285 V, Jp1=124.08 mA/cm2; Ep2= 0.354 V, Jp2=116.74 mA/cm2; in curve (b), E'p1=-0.342 V, J'p1= 81.65 mA/cm2; E'p2=0.355 V, J'p2=116.75 mA/cm2. This indicates that the addition of H2O2 in H2SO4 solution affects the first electro-oxidation step of Sn, causing Ep1 to move negatively and Jp1 to decrease obviously, but its effect on the second electro-oxidation step of Sn is not obvious.
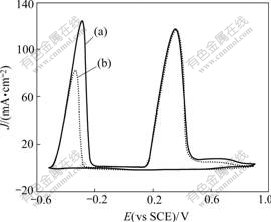
Fig.1 Cyclic voltammograms of Sn/PANI electrode at scan rate of 50 mV/s in various solutions: (a) 0.5 mol/L H2SO4; (b) 0.2 mol/L H2O2+0.5 mol/L H2SO4
Fig.2 shows the Raman spectra of the Sn/PANI electrode after being electro-oxidized in 0.2 mol/L H2O2 +0.5 mol/L H2SO4 (Fig.2(a)) and in 0.5 mol/L H2SO4 (Fig.2(b)), respectively. Compared the Raman spectrum of the electrode after being electro-oxidized with that of the pure PANI electrode shown in Fig.2(a), all the relative intensities of PANI electrode’s Raman bands are reduced obviously, except for the Raman band at 579.9 cm-1, corresponding to the Raman band of Sn(OH)4 at 580 cm-1[14], which may suggest that Sn hydroxide covering on this PANI film is mainly composed of Sn(OH)4. Similar phenomenon can be observed in Fig.2(b), but the position of Raman band at 579.9 cm-1 in Fig.2(a) moved negatively to 583.2 cm-1, existing a little warp compared with the Raman band of Sn(OH)4. So the component of Sn hydroxide covering on this PANI film may be more complicated.
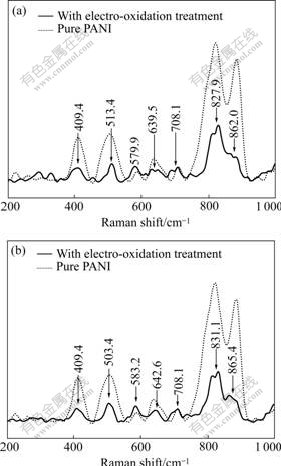
Fig.2 Comparison of surface Raman spectra of Sn/PANI electrode after being electro-oxidized in various solutions: (a) PANI electrode and electrode electro-oxidized in 0.2 mol/L H2O2+0.5 mol/L H2SO4; (b) PANI electrode and electrode electro-oxidized in 0.5 mol/L H2SO4
3.2 Electrocatalytic activities of Pt/Sn hydroxide/ PANI electrodes for formaldehyde oxidation
Fig.3 shows typical cyclic voltammograms for electro-oxidation of formaldehyde on Pt/Sn hydroxide/ PANI electrodesⅠ, Ⅱ and Pt/Sn/PANI electrode. Two peaks of electro-oxidation of formaldehyde can be observed obviously, which were also observed in Ref.[15]. In the positive sweep direction, current peaks in curves 1, 2 and 3 are observed at 0.772, 0.744 and 0.726 V (peakⅠ, peakⅠ, peakⅠ ), respectively. In the negative sweep direction, current peaks in curves 1, 2 and 3 are also observed at 0.578, 0.542 and 0.577 V (peak Ⅱ, peak Ⅱ and peak Ⅱ ), respectively. The current density of peak Ⅰ is about 1.252 times as large as that of peak Ⅰ and is about 2.832 times as large as that of peakⅠ. The current density of peak Ⅱ is about 1.422 times as large as that of peak Ⅱ and is about 2.9 times as large as that of peak Ⅱ . This implies that the electro-oxidation treatment of Sn can improve the electrocatalytic activity of the Pt/Sn/PANI electrode for the oxidation of formaldehyde obviously. It is known that electro-oxidation of formaldehyde on Pt catalyst is a self-poisoning reaction which blocks the active electrocatalyst surface by strongly adsorbed reaction intermediates such as CO, thus diminishing the electrocatalytic activity by poisoning the catalyst. VILLULLAS and MATTOSCOSTA[8] and SANTOS and PROFETI[9] reported that there may exist a bifunctional mechanism between SnO2 and Pt. In this mechanism, hydroxy species formed during the polarization process of the electrodes would be adsorbed on SnO2 and form SnO2—(?OH) which can combine with the adsorbed CO and depress the catalyst’s poisoning. So possibly there is similar effect between the quadrivalent Sn hydroxide and Pt, which upgrades the Pt/Sn/PANI electrode’s electrocatalytic activity. H2O2 can affect the electro-oxidation behavior of metal (such as Sn) in the solution and is beneficial to the formation of metal hydroxide (such as Sn(OH) 4)[16], which may result in difference in the electrocatalytic activity between Pt/Sn hydroxide/ PANI electrodeⅠand Pt/Sn hydroxide/PANI electrode Ⅱ. Moreover, it is found from curve 3 that Sn on Pt/Sn/PANI electrode is not electro-oxidized in H2SO4 solution. This may be resulted from the fact that the existence of Pt suppresses the electro-oxidation of Sn. According to Ref.[9], the addition of Sn with Pt in the form of an alloy or ad-atom results in the promotion of the catalyst performance. But in this work, the Pt/Sn/PANI electrode was prepared by depositing Pt onto Sn layer which was on the PANI film. And Sn did not alloy with Pt or combine as ad-atom. So Sn cannot promote the Pt catalyst performance when formaldehyde is electro-oxidized on the Pt/Sn/PANI electrode.

Fig.3 Cyclic voltammograms of Pt/Sn hydroxide/PANI electrodesⅠ(curve 1), Ⅱ(curve 2) and Pt/Sn/PANI electrode (curve 3) in 0.8 mol/L HCHO+0.5 mol/L H2SO4 at scan rate of 50 mV/s
3.3 Effects of Qdep (deposition charge) of Sn and Pt on electrocatalytic activities of Pt/Sn hydroxide/ PANI electrodes
The relationship between Qdep of Sn and the forward peak current density(Jp) of CV on Pt/Sn hydroxide/PANI electrodeⅠ(curve 1) and Pt/Sn hydroxide/PANI electrode Ⅱ(curve 2) in 0.8 mol/L HCHO+0.5 mol/L H2SO4 is shown in Fig.4 (Qdep of PANI, 96 mC/cm2; Qdep of Pt, 600 mC/cm2; scan rate, 50 mV/s). It can be seen from curve 1 in Fig.4 that Jp approximatively linearly increases with Qdep of Sn ranging from 240 to 945 mC/cm2, and then drops slowly after Qdep>945 mC/cm2. Similarly, in curve 2, Jp approximatively linearly increases with Qdep of Sn ranging from 240 to 1 215 mC/cm2, and then drops slowly after Qdep>1 215 mC/cm2. This may be explained as follows: when Qdep is low (Qdep<945 mC/cm2 for electrodeⅠor Qdep<1 215 mC/cm2 for electrode Ⅱ), the Sn hydroxide particles disperse uniformly on the surface of nanofibrous PANI film and show good assistant effect on the electrocatalytic performance of the catalyst. So Jp increases with the increase of Qdep of Sn. However, for higher Qdep of Sn (Qdep>945 mC/cm2 for electrodeⅠor Qdep>1 215 mC/cm2 for electrode Ⅱ), some Sn particles are deposited on the surface of Sn particles deposited
Fig.4 Effect of Qdep of Sn on electrocatalytic activity of Pt/Sn hydroxide/PANI electrodesⅠ(curve 1) and Ⅱ(curve 2)
already and the accumulation of metal catalyst occurs[17], which results in the decrease of the valuable specific surface area of catalyst for electro-oxidation of formaldehyde and the electrocatalytic activity of the electrodes.
The relationship between Qdep of Pt and Jp of CV on Pt/Sn hydroxide/PANI electrodeⅠ(curve 1) and Pt/Sn hydroxide/PANI electrode Ⅱ(curve 2) in 0.8 mol/L HCHO+0.5 mol/L H2SO4 is shown in Fig.5 (Qdep of PANI, 96 mC/cm2; Qdep of Sn on Pt/Sn hydroxide/PANI electrodeⅠ, 945 mC/cm2; Qdep of Sn on Pt/Sn hydroxide/ PANI electrode Ⅱ, 1 215 mC/cm2; scan rate, 50 mV/s). It can be seen that in curve 1, Jp approximatively linearly increases with Qdep of Pt ranging from 180 to 750 mC/cm2, and then tends to stabilize gradually after Qdep>750 mC/cm2. Similarly, in curve 2 Jp approximatively linearly increases with Qdep of Pt ranging from 180 to 600 mC/cm2, and then tends to stabilize gradually after Qdep>600 mC/cm2. This may be explained as follows: when Qdep of Pt is low (Qdep<750 mC/cm2 for electrodeⅠor Qdep<600 mC/cm2 for electrode Ⅱ), Pt particles disperse uniformly on the surface of the electrode, so Jp increases with the increase of Qdep of Pt. However, for higher Qdep of Pt, more and more Pt particles are deposited on the surface of Pt particles deposited on the electrode already and the accumulation of metal catalyst occurs[17], but the valuable specific surface area of Pt does not increase, so the electrocatalytic activities of the electrodes tend to stabilize gradually.
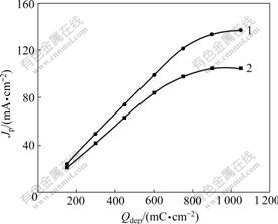
Fig.5 Effect of Qdep of Pt on electrocatalytic activity of Pt/Sn hydroxide/PANI electrodesⅠ(curve 1) and Ⅱ(curve 2)
3.4 Effect of Qdep of PANI on electrocatalytic activity of Pt/Sn hydroxide/PANI electrodeⅠ
The relationship between Qdep of PANI and Jp of CV on Pt/Sn hydroxide/PANI electrodeⅠin 0.8 mol/L HCHO+0.5 mol/L H2SO4 is shown in Fig.6 (Qdep of Sn, 945 mC/cm2; Qdep of Pt, 600 mC/cm2; scan rate, 50 mV/s). It can be seen that Jp reaches the maximum when Qdep of PANI is 102 mC/cm2. Nanofibrous PANI supporter offers effective electro-active sites to the catalysts with high dispersion degree. But with the increase of PANI film thickness, some Pt particles may be deposited into the PANI films and cannot exert effective electrocatalytic effect on the electro-oxidation of formaldehyde, so there is an appropriate scope for Qdep of PANI.
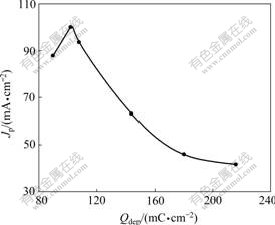
Fig.6 Effect of Qdep of PANI on electrocatalytic activity of Pt/Sn hydroxide/PANI electrodeⅠ
3.5 Effects of formaldehyde concentration of CV and scan rate on electrocatalytic activity of Pt/Sn hydroxide/PANI electrodeⅠ
The relationship between formaldehyde concentration and Jp of CV on Pt/Sn hydroxide/PANI electrodeⅠis shown in Fig.7 (Qdep of PANI, 96 mC/cm2; Qdep of Sn, 945 mC/cm2; Qdep of Pt, 600 mC/cm2; scan rate, 50 mV/s). It can be seen from Fig.7 that Jp increases with the increase of formaldehyde concentration and a plateau reaches gradually after formaldehyde concentration of 2 mol/L.
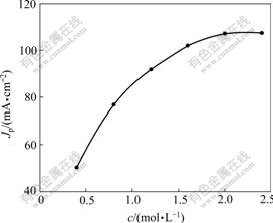
Fig.7 Effect of formaldehyde concentration on electrocatalytic activity of Pt/Sn hydroxide/PANI electrodeⅠ
The relationship between the square root of the CV scan rate (v1/2) and Jp of CV on Pt/Sn hydroxide/PANI electrodeⅠin 0.8 mol/L HCHO+0.5 mol/L H2SO4 is shown in Fig.8 (Qdep of PANI, 96 mC/cm2; Qdep of Sn, 945 mC/cm2; Qdep of Pt, 600 mC/cm2). It can be seen from Fig.8 that a good linear relationship between v1/2 and Jp can be observed (R=0.999 6). The research results suggest that the electro-oxidation of formaldehyde on Pt/Sn hydroxide/PANI electrodeⅠmay be mainly affected by the diffusion process of formaldehyde.
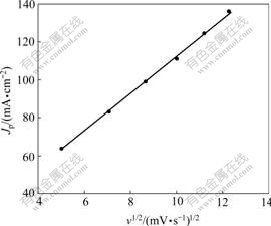
Fig.8 Effect of CV scan rate on electrocatalytic activity of Pt/Sn hydroxide/PANI electrodeⅠ
4 Conclusions
1) The CVs of the Sn/PANI electrode in 0.5 mol/L H2SO4 and in 0.2 mol/L H2O2+0.5 mol/L H2SO4 show that H2O2 added in H2SO4 solution affects the first electro-oxidation peak of Sn, but its effect on the further electro-oxidation of Sn is not obvious. The Raman spectrum measurement results indicate that the Sn hydroxide covering on the Sn/PANI electrode electro- oxidized in 0.2 mol/L H2O2+0.5 mol/L H2SO4 is mainly composed of Sn(OH)4, and the component of the Sn hydroxide covering on the Sn/PANI electrode electro- oxidized in 0.5 mol/L H2SO4 is more complicated.
2) The electrocatalytic activities of Pt/Sn hydroxide/ PANI electrodesⅠand Ⅱ and Pt/Sn/PANI electrode are investigated by CV method. The corresponding results show that Pt/Sn hydroxide/PANI electrodesⅠand Ⅱ exhibit higher electrocatalytic activity for electro- oxidation of formaldehyde than Pt/Sn/PANI electrode, and Pt/Sn hydroxide/PANI electrodeⅠgets the highest electro-catalytic activity for the electro-oxidation of formaldehyde. The electro-oxidation of formaldehyde on Pt/Sn hydroxide/PANI electrodeⅠmay be mainly affected by the diffusion process of formaldehyde.
3) The best Qdep of PANI is 102 mC/cm2. For Pt/Sn hydroxide/PANI electrodeⅠ, the best Qdep of Sn is 945 mC/cm2, and the electrocatalytic activity for the electro-oxidation of formaldehyde tends to stabilize gradually after Qdep of Pt is higher than 750 mC/cm2. For Pt/Sn hydroxide/PANI electrode Ⅱ, the best Qdep of Sn is 1 215 mC/cm2 and the electrocatalytic activity for the electro-oxidation of formaldehyde tends to stabilize gradually after Qdep of Pt is higher than 600 mC/cm2.
References
[1] VIELSTICH W. Electrochemical energy conversion-methanol fuel cell as example [J]. Journal of the Brazilian Chemical Society, 2003, 14(4): 503-509.
[2] LI Xi, CAO Guang-yi, ZHU Xin-jian, WEI Dong. Identification and analysis based on genetic algorithm for proton exchange membrane fuel cell stack [J]. Journal of Central South University of Technology, 2006, 13(4): 428-431.
[3] LI Song-lin, LIU Yi, CUI Jian-min, YANG Wen-zhi, LI Hao-peng, HE Yi-lun. Synthesis and hydrogen desorption properties of Mg2FeH6 hydrogen storage material by reactive mechanical alloying [J]. Journal of Central South University: Science and Technology, 2008, 39(1): 428-431. (in Chinese)
[4] IWASITA T. Electrocatalysis of methanol oxidation [J]. Electrochimica Acta, 2002, 47(22/23): 3663-3674.
[5] G?MEZ J L, MART?NEZ-HUERTA M V, ROJAS S. Methanol electrooxidation on Pt-Ru nanoparticles supported on functionalised carbon black [J]. Catalysis Today,2006, 116(3):422-432.
[6] COLMATI F, ANTOLINI E, ERNESTO R. Pt-Sn/C electrocatalysts for methanol oxidation synthesized by reduction with formic acid[J]. Electrochimica Acta, 2005, 50(28): 5496-5503.
[7] JAYARAMAN S, JARAMILLO T F, BAECK S H, MCFARLAND E W. Synthesis and characterization of Pt-WO3 as methanol oxidation catalysts for fuel cells [J]. Journal of Physical Chemistry (Part B), 2005, 109(48): 22958-22966.
[8] VILLULLAS H M, MATTOSCOSTA F I. Anodic oxidation of formaldehyde on Pt-modified SnO2 thin film electrodes prepared by a sol-gel method [J]. Electrochimica Acta, 2004, 49(22/23): 3909-3916.
[9] SANTOS A L, PROFETI D. Electrooxidation of methanol on Pt microparticles dispersed on SnO2 thin films [J]. Electrochimica Acta, 2005, 50(13): 2615-2621.
[10] MERCEDES H, VILLULLAS H M, MATTOS-COSTA F. Electrochemical oxidation of methanol on Pt nanoparticles dispersed on RuO2 [J]. Journal of Physical Chemistry (Part B), 2004, 108(34): 12898-12903.
[11] CHEN A, RUSSA D J, MILLER B. Effect of the iridium oxide thin film on the electrochemical activity of platinum nanoparticles [J]. Langmuir, 2004, 20(22): 9695-9702.
[12] ZHOU Hai-hui, JIAO Shu-qiang, CHEN Jin-hua, WEI Wan-zhi, KUANG Ya-fei. The electrocatalytic activity of Pt-modified nanofibers PANI electrode for methanol oxidation [J]. Acta Physico-Chimica Sinic, 2004, 20(1): 9-14. (in Chinese)
[13] JIAO Shu-qiang, PENG Xia-hui, ZHOU Hai-hui, CHEN Jin-hua, KUANG Ya-fei. The electrosynthesis of PANI by pulse galvanostatic method [J]. Chemical Journal of Chinese Universities, 2003, 24(6): 1118-1121. (in Chinese)
[14] HUANG B X, TORNATORE P, LI Y S. IR and Raman spectroelectrochemical studies of corrosion films on tin [J]. Electrochimica Acta, 2001, 46(5): 671-679.
[15] OKAMOTO H, KON W, MUKOUYAMA Y. Five current peaks in voltammograms for oxidations of formic acid, formaldehyde and methanol on platinum [J]. Journal of Physical Chemistry (Part B), 2005, 109(32): 15659-15666.
[16] CHANGA S T, LEUB I C. Electrodeposition of nanocrystalline SnO2 coatings with two-layer microstructure [J]. Journal of Crystal Growth, 2004, 273(2): 195-202.
[17] YE J H, FEDKIW P S. Electrodeposition of high-surface area platinum in a well adherent Nafion film on glassy carbon [J]. Electrochimica Acta, 1996, 41(2): 221-231.
(Edited by CHEN Wei-ping)
Foundation item: Project(50473022) supported by the National Natural Science Foundation; Projects(05FJ3080, 2006FJ4100) supported by the Science and Technology Program of Hunan Province, China; Project(20060400874) supported by the Postdoctoral Foundation of China; Project (2007018) supported by the Foundation of State Key Laboratory of Chemo/Biosensing and Chemometrics of China; Project(2006) supported by the Postdoctoral Foundation of Hunan University
Received date: 2008-01-08; Accepted date: 2008-04-11
Corresponding author: KUANG Ya-fei, Professor; Tel: +86-731-8821874; E-mail: yafeik@163.com