J. Cent. South Univ. Technol. (2008) 15: 531-534
DOI: 10.1007/s11771-008-0100-1
Preparation of LiFePO4 for lithium ion battery using Fe2P2O7 as precursor
HU Guo-rong(胡国荣), XIAO Zheng-wei(肖政伟), PENG Zhong-dong(彭忠东),
DU Ke(杜 柯), DENG Xin-rong(邓新荣)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract:In order to obtain a new precursor for LiFePO4, Fe2P2O7 with high purity was prepared through solid phase reaction at 650 ℃ using starting materials of FeC2O4 and NH4H2PO4 in an argon atmosphere. Using the as-prepared Fe2P2O7, Li2CO3 and glucose as raw materials, pure LiFePO4 and LiFePO4/C composite materials were respectively synthesized by solid state reaction at 700 ℃ in an argon atmosphere. X-ray diffractometry and scanning electron microscopy(SEM) were employed to characterize the as-prepared Fe2P2O7, LiFePO4 and LiFePO4/C. The as-prepared Fe2P2O7 crystallizes in the 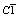 space group and belongs to β-Fe2P2O7 for crystal phase. The particle size distribution of Fe2P2O7 observed by SEM is 0.4-3.0 μm. During the Li+ ion chemical intercalation, radical
space group and belongs to β-Fe2P2O7 for crystal phase. The particle size distribution of Fe2P2O7 observed by SEM is 0.4-3.0 μm. During the Li+ ion chemical intercalation, radical  is disrupted into two
is disrupted into two 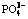 ions in the presence of O2-, thus providing a feasible technique to dispose this poor dissolvable pyrophosphate. LiFePO4/C composite exhibits initial charge and discharge capacities of 154 and 132 mA·h/g, respectively.
ions in the presence of O2-, thus providing a feasible technique to dispose this poor dissolvable pyrophosphate. LiFePO4/C composite exhibits initial charge and discharge capacities of 154 and 132 mA·h/g, respectively.
Key words: lithium ion battery; cathode material; preparation; precursor; LiFePO4; Fe2P2O7
1 Introduction
The original work of PADHI et al[1] for LiFePO4 has aroused researchers’ interest in this new cathode material for lithium ion batteries. With the advantages of cheap raw materials, environmental friendliness, high safety, good cycle stability and appreciable specific capacity of 170 mA·h/g, LiFePO4 has established itself as a potent competitor in cathode material field and challenged the widely used LiCoO2 and other cathode material candidates such as LiMn2O4, LiMnO2 and LiNiO2[2-4]. In particular based on the listed characteristics, LiFePO4 has highly potential application in hybrid electric vehicles(HEVs)[5].
Presently, the work of the preparation of LiFePO4 has centered on different synthetic methods: solid state reactions at high temperature[6], hydrothermal synthesis[7] and sol-gel methods[8] etc. In most cases, lithium salts or lithium hydroxide, ferrous or ferric compounds and phosphates are used as raw materials to obtain LiFePO4 either in solid or liquid state[9-11]. Here, enlightened by the concept of precursor Ni1/3Co1/3Mn1/3CO3 with molar ratio n(Ni)?n(Mn)?n(Co)= 1?1?1 for LiNi1/3Co1/3Mn1/3O2, a new precursor Fe2P2O7 for LiFePO4 was presented. The compound has the following characteristics.
1) Besides O2-, the component includes only Fe and P with molar ratio of 1?1.
2) The valences of elements Fe and P in Fe2P2O7 are the same as those in LiFePO4, namely +2 and +5 respectively, and during the synthesis of LiFePO4 they stay unchanged.
In this work, the precursor Fe2P2O7 with high purity was prepared and then chemically intercalated with Li+ ion to successfully synthesize LiFePO4 through solid state reaction.
2 Experimental
Fe2P2O7 was prepared by a solid state reaction using FeC2O4 and NH4H2PO4 as raw materials. The detailed procedure was as follows: the stoichiometric amount of raw materials FeC2O4 and NH4H2PO4 were mixed and milled in a planetary mill at a speed of 300 r/min for 4 h in acetone to get them thoroughly mixed. The slurry was then dried at 80 ℃ to remove acetone. The dried mixture was heated to 650 ℃ in an argon atmosphere to start the reaction of FeC2O4 and NH4H2PO4, and finally Fe2P2O7 was prepared.
Fe2P2O7, stoichiometric Li2CO3 and some glucose were ball-milled at a speed of 300 r/min for 4 h in acetone to ensure a homogenous mixing. After being dried at 80 ℃ overnight, the mixture was ground and then sintered at 700 ℃ under a flowing argon gas. Composite cathode material LiFePO4/C(simply denoted as LiFePO4/C) was synthesized in the sintering procedure. Carbon content of LiFePO4/C was determined as follows: dissolve LiFePO4/C in hydrochloric acid, filtrate the solution, wash the indissoluble carbon thoroughly, finally calculate mass fraction of the dried carbon.
X-ray powder diffraction of Fe2P2O7 and LiFePO4 was performed on Philips X’pert powder diffractometer with Cu Kα radiation. The morphology of Fe2P2O7 was characterized using scanning electron microscope(SEM) (JSM-5600LV, JEOL).
The electrochemical performance test of LiFePO4/C was carried out with coin cell 2025 which was assembled in an argon atmosphere glove-box. The electrolyte was 1 mol/L LiPF6 in a 1?1 (volume ratio) mixture of ethylene carbonate(EC) and dimethyl carbonate(DEC). Membrane used is Celgard2300 and the metal lithium was used as the anode electrode. N-methyl pyrrolidinone was used as organic solvent, and the cathode electrode was made, which consisted of 80% LiFePO4, 10% PVDF (polyvinylidence fluoride) binder and 10% carbon black (in mass fraction) coated onto an aluminum foil current collector. The cathode was dried under vacuum at 120 ℃ for 24 h before cell assembly procedure. The coin cells were galvanostatically charged and discharged at 0.1C rate and cut-off of 2.5-4.1 V.
3 Results and discussion
3.1 Preparation and characterization of Fe2P2O7
The preparation of Fe2P2O7 using FeC2O4 and NH4H2PO4 as raw materials in an argon atmosphere can be described as follows:
2NH4H2PO4+2FeC2O4→
Fe2P2O7+2NH3↑+3H2O↑+2CO2↑+ 2CO↑ (1)
Actually, the divalent metal pyrophosphates M2P2O7 (M=Fe, Mn, Co, Ni, Cu, Zn) are polymorphic, including α, β and γ phases. Radical 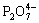 is the common ion in pyrophosphates, among which the P—O—P bond angles are different from each other in the range of 120?-180?, and the bond length of P—O in P—O—P is longer than that in tip P—O (Fig.1). HOGGINS[12] reported that P—O—P bond angle of Fe2P2O7 is linear as a result of extensive p bonding with the p orbital on the bridge oxygen atom.
is the common ion in pyrophosphates, among which the P—O—P bond angles are different from each other in the range of 120?-180?, and the bond length of P—O in P—O—P is longer than that in tip P—O (Fig.1). HOGGINS[12] reported that P—O—P bond angle of Fe2P2O7 is linear as a result of extensive p bonding with the p orbital on the bridge oxygen atom.
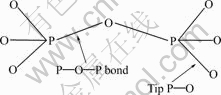
Fig.1 Plane configuration of structure of radical
The X-ray diffraction pattern of Fe2P2O7 prepared is shown in Fig.2. According to Ref.[13], the as-prepared Fe2P2O7 crystallizes in the  space group and belongs to β-Fe2P2O7 for crystal phase, which is isostructural with high temperature form of β-Mg2P2O7.
space group and belongs to β-Fe2P2O7 for crystal phase, which is isostructural with high temperature form of β-Mg2P2O7.
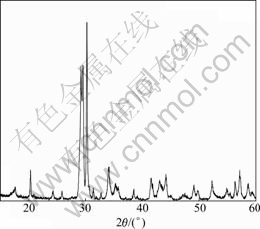
Fig.2 X-ray diffraction pattern of Fe2P2O7 prepared at 650 ℃
In this experiment, fully crystallized Fe2P2O7 was made at 700-900 ℃ using FeC2O4 and NH4H2PO4 as raw materials in an argon atmosphere. Here, it must be mentioned that according to the experiment results, there is, to a certain extent, no difference in electrochemical performance between LiFePO4/C samples with same carbon content using either Fe2P2O7 prepared at 650 ℃ as precursor or fully crystallized ones obtained at higher temperature.
The SEM image of the as-prepared Fe2P2O7 is shown in Fig.3. The powders have a narrow particle size distribution ranging from 0.4 to 3.0 μm. An average primary crystal size can be roughly estimated using Schererr equation[14]:
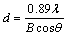 (2)
(2)
where d is the average crystal size, 0.89 is Schererr constant, λ=0.154 nm is the wavelength of X-ray, B is the width (in radian) of the X-ray diffraction peak at half maximum intensity and θ is Bragg diffraction angle. With the reference of the X-ray diffraction data, the calculated crystal size is 91 nm. The small primary crystallinity and fine particles of the as-prepared Fe2P2O7 allow its easily homogenous mixing and reaction with Li2CO3, ensuring a thorough Li+ chemical intercalation in the synthesis procedure of LiFePO4.

Fig.3 SEM image of Fe2P2O7 powder prepared at 650 ℃ using FeC2O4 and NH4H2PO4 as raw materials
3.2 Synthesis of LiFePO4 and mutual transformation between PO43- and P2O74-
The synthesis of LiFePO4 using Fe2P2O7 and Li2CO3 as raw materials in an inert atmosphere can be expressed by the following reaction:
Fe2P2O7+Li2CO3→2LiFePO4+CO2↑ (3)
It should be noticed that in the procedure of synthesis of Fe2P2O7 and thereafter LiFePO4 an exciting mutual conversion between  and
and 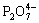 is realized:
is realized:  →
→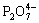 →
→ In this work,
In this work,  is converted into
is converted into 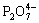 in the reaction of NH4H2PO4 and FeC2O4. There are some other cases for this conversion. Generally, Fe2P2O7 is prepared by the reduction of FePO4 in a reductive atmosphere containing H2 via a long and strict process[15]. Some divalent metal pyrophosphates can be obtained by heating their corresponding ammoniated or acidic phosphates. For example:
in the reaction of NH4H2PO4 and FeC2O4. There are some other cases for this conversion. Generally, Fe2P2O7 is prepared by the reduction of FePO4 in a reductive atmosphere containing H2 via a long and strict process[15]. Some divalent metal pyrophosphates can be obtained by heating their corresponding ammoniated or acidic phosphates. For example:
2NH4NiPO4?6H2O→Ni2P2O7+2NH3↑+13H2O (4)
In aqueous solution, dilute phosphoric acid H3PO4 becomes viscid when heated to 422 K and then dehydrates into H4P2O7 when heated to 485-486 K, and finally pure H4P2O7 is obtained at 528-533 K.
For the transformation from 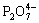 to
to  however, there are few cases reported ever. In this work, pure LiFePO4 was prepared by disrupting the
however, there are few cases reported ever. In this work, pure LiFePO4 was prepared by disrupting the 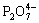 radical into two
radical into two  ions in the presence of O2- at elevated temperature in an inert atmosphere. Though Fe2P2O7 can be dissolved in acidic solution, it is sluggish in kinetics. Due to the high dissolubility of LiFePO4 in acidic solution, the structural conversion from
ions in the presence of O2- at elevated temperature in an inert atmosphere. Though Fe2P2O7 can be dissolved in acidic solution, it is sluggish in kinetics. Due to the high dissolubility of LiFePO4 in acidic solution, the structural conversion from 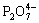 to
to  provides a feasible technique to dispose those pyrophosphates of low dissolubility.
provides a feasible technique to dispose those pyrophosphates of low dissolubility.
3.3 XRD characterization of pure LiFePO4 and LiFePO4/C
The X-ray powder diffraction patterns of pure LiFePO4 prepared using the as-prepared Fe2P2O7 and Li2CO3 and LiFePO4/C obtained using Fe2P2O7, Li2CO3 and glucose are shown in Fig.4. The two diffraction lines are indexed to an orthorhombic crystal structure (space group Pnma). The peaks in both curves are very sharp and perfect, suggesting a high degree of crystallinity. The patterns agree well with that of phospho-olivine LiFePO4, and no impurity phase is detected. For the diffraction of LiFePO4/C, no peaks of carbon are revealed, indicating the amorphous property of the electron conductor.

Fig.4 X-ray diffraction patterns of pure LiFePO4 (a) and LiFePO4/C (b)
3.4 Electrochemical test of LiFePO4/C
The charge/discharge curves of LiFePO4/C (Fig.5) reveal a flat potential plateau of 3.45 V (vs Li+/Li). At this voltage, the lithium extraction/insertion proceeds at the room-temperature in a two-phase reaction between LiFePO4 and FePO4 that belongs to the same space group. A 0.08 V voltage difference between charge and discharge plateaus is shown in Fig.5, which represents very small polarization in electrodes and electrolyte of coin cell during charge and discharge process.
The first charge and discharge capacities of LiFePO4/C are 154 and 132 mA·h/g, accounting for 90.6% and 77.6% of the theoretical value (170 mA·h/g), respectively. Here, it can be concluded the ecumenic electrochemical performance and low efficiency of the initial cycle (85.7%) of the LiFePO4/C are ascribed to the non-optimal coating of carbon and calcining temperature and time. Furthermore, the appearance of LiFePO4/C is a medium to dark grey, and according to Ref.[16], the conductivity of the as-prepared LiFePO4/C in this color is not high enough to ensure an appreciable electrochemical performance. More evidence about the poor conductivity of LiFePO4/C is the low efficiency of the initial charge/discharge. Further work will be carried out to study charge/discharge capacity and improve initial charge/discharge efficiency of LiFePO4/C.

Fig.5 Initial charge/discharge curves of LiFePO4/C (mass fraction of carbon is 9%)
4 Conclusions
1) β-Fe2P2O7 with high purity is prepared through solid phase reaction at 650 ℃ using starting materials of FeC2O4 and NH4H2PO4 in an argon atmosphere. Pure LiFePO4 and LiFePO4/C can be synthesized by solid state reaction at 700 ℃ in an argon atmosphere using the as-prepared Fe2P2O7 and Li2CO3.
2) Radical 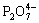 is disrupted into two
is disrupted into two  ions in the presence of O2- during the synthesis procedure of LiFePO4, thus an important mutual transformation is realized:
ions in the presence of O2- during the synthesis procedure of LiFePO4, thus an important mutual transformation is realized:  →
→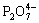 →
→ which offers a feasible technique to deal with this hardly dissolvable pyrophosphate.
which offers a feasible technique to deal with this hardly dissolvable pyrophosphate.
3) Composite material LiFePO4/C shows appreci- able initial charge and discharge capacities of 154 and 132 mA·h/g, respectively.
References
[1] PADHI A K, NANJUNDASWAMY K S, MASQUELIER C, OKADA S, GOODENOUGH J B. Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates [J]. Journal of Electrochemical Society, 1997, 144(5): 1609-1613.
[2] ZHANG Bao, LI Xin-hai, ZHU Bing-quan. Low temperature synthesis and electrochemical properties of LiFePO4/C cathode [J]. Journal of Central South University: Science and Technology, 2006, 37(3): 505-508. (in Chinese)
[3] JOONGPYO S, KATHYN A S. Cycling performance of low-cost lithium ion batteries with natural graphite and LiFePO4 [J]. Journal of Power Source, 2003, 119/121: 955-958.
[4] BELHAROUAK I, JOHRSON C, AMINE K. Synthesis and electrochemical analysis of vapor-deposited carbon-coated LiFePO4 [J]. Electrochemistry Communications, 2005, 7(10): 983-988.
[5] STRIEBEL K, SHIM J, SIERRA A. The development of low cost LiFePO4-based high power lithium-ion batteries [J]. Journal of Power Source, 2005, 146(1/2): 33-38.
[6] SCACCIA S, CAREWSKA M, WISNIEWSKI P, PROSINI P P. Morphological investigation of sub-micron FePO4 and LiFePO4 particles for rechargeable lithium batteries [J]. Materials Research Bulletin, 2003, 38(7): 1155-1163.
[7] YANG S F, ZAVALIJ P Y, STANLEY W M. Hydrothermal synthesis of lithium iron phosphate cathodes [J]. Electrochemistry Communication, 2001, 3(9): 505-508.
[8] HU Y Q, DOEFF M M, KOSTECKI R, FINONES R. Electro- chemical performance of sol-gel synthesized LiFePO4 in lithium batteries [J]. Journal of Electrochemical Society, 2004, 151(8): A1279-A1285.
[9] AMINE K, LIU J, BELHAROUAK I. High-temperature storage and cycling of C-LiFePO4/graphite Li-ion cells [J]. Electrochemistry Communication, 2005, 7(7): 669-673.
[10] SINGHAL A, SKAMDAN G, AMATUCCI G, BADWAY F, YE N, MANTHIRAM A, YE H, XU J J. Nanostructured electrodes for next generation rechargeable electrochemical devices [J]. Journal of Power Source, 2004, 129(1): 38-44.
[11] ALAN D S, GIRTS V, JOHN R O. A solution-precursor synthesis of carbon-coated LiFePO4 for Li-ion cells [J]. Journal of Electrochemical Society, 2005, 152(12): A2376-A2382.
[12] HOGGINS J T, SWINNEA J S, STEINFINK H. Crystal structure of Fe2P2O7 [J]. Journal of Solid State Chemistry, 1983, 47(3): 278-283.
[13] PARADA C, PERLES J, SAEZ-PUCHE R, RUIZ-VALERO C, SNEJKO N. Crystal growth, structure, and magnetic properties of a new polymorph of Fe2P2O7 [J]. Chemistry Material, 2003, 15(17): 3347-3351.
[14] WU Da-xiong, WU Xi-jun, LU Yan-fei, WANG Hui. Synthesis and room temperature conductivity of nano-LaF3 bulk material [J]. Trans Nonferrous Met Soc China, 2006,16(4): 828-832.
[15] MAMORU A, KYOJI O. Effects of the method of preparing iron orthophosphate catalyst on the structure and the catalytic activity [J]. Applied Catalysis A: General, 1999, 180(1/2): 47-52.
[16] CHUNG S Y, BLOCKING J T, CHIANG Y M. Electronically conductive phospho-olivines as lithium storage electrodes [J]. Nature Material, 2002, 1(2): 123-128.
Foundation item: Project(50604018) supported by the National Natural Science Foundation of China
Received date: 2007-12-18; Accepted date: 2008-03-09
Corresponding author: HU Guo-rong, Professor, PhD; Tel: +86-731-8830474; E-mail: hgrhsj@263.net
(Edited by CHEN Wei-ping)