
Ternary compounds and solid-state phase equilibria in Mg-rich side of Mg-Zn-Ca system at 300 ℃
LI Hong-xiao, REN Yu-ping, MA Qian-qian, JIANG Min, QIN Gao-wu
Key Laboratory for Anisotropy and Texture of Materials, Ministry of Education,
Northeastern University, Shenyang 110819, China
Received 30 October 2010; accepted 27 May 2011
Abstract: The phase equilibria and compositions in Mg-rich side at 300 °C were investigated in Mg-Zn-Ca ternary system through the equilibrated alloy method by using scanning electron microscopy, electron probe microanalysis, X-ray diffraction and transmission electron microscopy. The results show that two ternary compounds T1 and T2 can be in equilibrium with the Mg-based solid solution in Mg-Zn-Ca system. T1 phase is a linear compound with the composition region (molar fraction) of 15% Ca, 20.5%-48.9% Zn and balanced Mg at 300 °C. Its hexagonal structure parameters decrease with increasing Zn content, i.e. a=0.992-0.945 nm, c=1.034-1.003 nm. T2 phase has hexagonal structure with the composition region of 26.4%-28.4% Mg, 63.2%-65.5% Zn and 7.1%-8.4% Ca. At 300 °C, the solubility of Zn in the Mg-based solid solution increases for the addition of Ca, the maximum solubility of Zn is 3.7%. Three-phase fields consisting of a-Mg+Mg2Ca+T1, a-Mg+T1+T2, a-Mg+T2+MgZn and MgZn+T2+Mg2Zn3 exist in the Mg-Zn-Ca system at 300 °C.
Key words: Mg-Zn-Ca system; ternary compound; structure; composition; phase equilibrium
1 Introduction
Alloying is the most effective method to modify the microstructures and properties of the structural Mg-based alloys. Zn addition to the magnesium alloy could result in the age-hardening [1-3]. Addition of Ca with low density and cost could increase the ignition temperature of the molten magnesium alloy due to the formation of the stable oxidization layer [4-5]. Specially, the ternary addition of Ca to the Mg-Zn alloys is more effective for the improvement of the mechanical properties [6-10].
As early as in the 1930s, research on the Mg-Zn-Ca system began. But till the 1960s, the partial isothermal section of the Mg-Zn-Ca system at 335 °C was obtained by CLARK [11]. Due to the limitation of the experimental methods and conditions, the information about the compounds and phase equilibria was not reliable. Recently, the Mg-Zn-Ca system has been thermodynamically assessed to develop a thermodynamic database for the further refinement of existing Mg-based alloys and the development of new ones. Because of the contradicting experimental data, the thermodynamic description of Mg-Zn-Ca system is not satisfied and the reliability of the calculated phase diagram need to be improved further [12-14].
Searching for the effective strengthening phases is the main aim of the magnesium alloy design. The solubility of compounds in the Mg-based solid solution and relative phase equilibrium relationships are the base of alloy design. Although ZHANG et al [15] reported the solubility range and crystal structure of the compound Ca3MgxZn15-x at 335 °C, there was limited information about the phase equilibria with Mg-based solid solution in the Mg-Zn-Ca system.
The solid-state phase equilibria between the Mg-based solid solution and the compounds are more important to understand the microstructure of Mg-Zn-Ca based alloys and to design appropriate aging processing. There is an eutectic reaction L α-Mg+Mg7Zn3 at 340 °C in the Mg-rich corner of Mg-Zn binary system[1]. Therefore, the purpose of the present work is to determine the phase equilibria at 300 °C in Mg-rich side of Mg-Zn-Ca system by means of the equilibrated alloy method. It is expected to provide more reliable thermodynamic parameters by using these equilibrium phase compositions to better understand the relationships among composition, microstructure and mechanical properties of Mg-Zn-Ca based alloys.
α-Mg+Mg7Zn3 at 340 °C in the Mg-rich corner of Mg-Zn binary system[1]. Therefore, the purpose of the present work is to determine the phase equilibria at 300 °C in Mg-rich side of Mg-Zn-Ca system by means of the equilibrated alloy method. It is expected to provide more reliable thermodynamic parameters by using these equilibrium phase compositions to better understand the relationships among composition, microstructure and mechanical properties of Mg-Zn-Ca based alloys.
2 Experimental
According to the Mg-Zn, Mg-Ca binary systems and some results of Mg-Zn-Ca ternary system [1, 11], a series of Mg-Zn-Ca alloys, Mg80Zn10Ca10 (nominal composition in molar fraction, %, so-called Zn10Ca10, the same as follows), Mg70Zn17Ca13, Mg53Zn32Ca15, Mg64Zn26Ca10, Mg50Zn40Ca10, Mg50Zn45Ca5 and Mg33Zn62Ca5 in the Mg-rich side, were designed in order to include all the compounds in equilibrium with the Mg-based solid solution. All the samples were prepared by using the high pure Zn (>99.999%), Mg (>99.99%) and Ca (>99.9%). The melting process was performed in the graphite crucible in an induction furnace under the protection of high pure Ar atmosphere. The mass of each ingot was about 30 g. All the samples cut from the ingots were wrapped with Ta foils and sealed in a quartz tube under vacuum of 10-2 Pa, then kept at 300 °C for 500 h, and finally quenched in water.
The phase constituents of heat-treated alloys were determined by X-ray diffractometer (XRD, Siemens D5000) with Cu Kα radiation, operating at a voltage of 40 kV and a current of 40 mA. The microstructure observation was carried out on SSX-500 scanning electron microscope with a voltage of 20 kV. The compositions of equilibrium phases in the alloys were analyzed on Shimadzu EPMA-1600 electron probe microanalyzer. The high pure Mg, Zn and CaCO3 were used as standard samples. Thin foils for transmission electron microscopy were prepared by ion beam thinning and then examined on Tecnai G2 20 operating at 200 kV.
3 Results and discussion
3.1 Relative phase equilibria with linear compound T1 at 300 °C
Among the alloys with Zn to Ca molar ratios of 1-4, the alloys Zn10Ca10 and Zn40Ca10 are in three-phase equilibrium; the alloys Zn17Ca13, Zn32Ca15 and Zn26Ca10 are in two-phase equilibrium at 300 oC. The equilibrium phase compositions in the alloys are listed in Table 1. It could be seen that the ternary compounds existing in these 5 alloys separately are with different Zn contents of 20.5%-48.9% (molar fraction, the same as follows) and almost the same Ca content (~15%). This characteristic of phase composition suggests that the ternary compounds mentioned above should belong to the same linear compound.
Table 1 Equilibrium phase constituents and compositions in Mg-Zn-Ca system at 300 °C
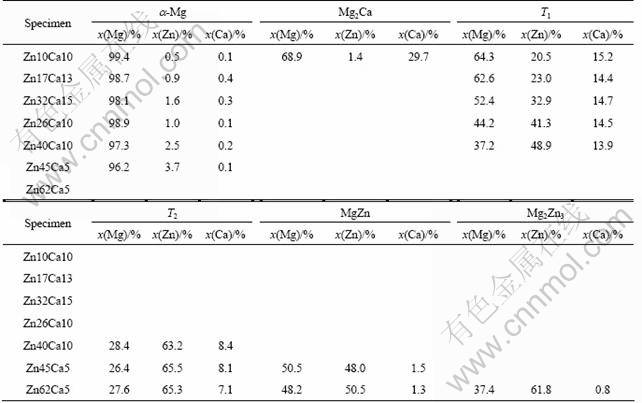
The microstructure of the Zn10Ca10 alloy in three-phase equilibrium at 300 °C is shown in Fig. 1(a). The crystal structure of the ternary compound with composition of 64.3Mg-20.5Zn-15.2Ca (so-called T1 phase) in the Zn10Ca10 alloy was analyzed by selected area electron diffraction (SAED) of transmission electron microscopy (TEM). The SAED pattern of [ ] zone axis of T1 phase is shown in Fig. 1(b), which suggests that T1 phase has hexagonal structure. Its structure parameters determined by XRD (Fig. 1(c)) are a=0.992 nm and c=1.034 nm.
] zone axis of T1 phase is shown in Fig. 1(b), which suggests that T1 phase has hexagonal structure. Its structure parameters determined by XRD (Fig. 1(c)) are a=0.992 nm and c=1.034 nm.

Fig. 1 Equilibrium microstructure of alloy Zn10Ca10 held at 300 °C (a), SAED pattern of [ ] zone axis of T1 phase (b) and corresponding XRD pattern (c)
] zone axis of T1 phase (b) and corresponding XRD pattern (c)
Besides the diffraction peaks caused by T1 phase, there also exist the diffraction peaks caused by Mg and Mg2Ca in the XRD pattern (Fig. 1(c)). Therefore, besides the binary compound Mg2Ca, the ternary compound T1 could also be in equilibrium with the Mg-based solid solution.
The equilibrium microstructures and corresponding XRD patterns of the alloys Zn17Ca13, Zn32Ca15 and Zn26Ca10 after equilibrium treatment at 300 °C are shown in Fig. 2. These alloys are in two-phase equilibrium. The diffraction peaks of Mg could be indexed, and all the other diffraction peaks could be indexed with hexagonal structure. The hexagonal structure parameters decrease continuously with the increase of Zn content in the ternary compounds. The structure parameters of the ternary compound with the composition of 62.6Mg-23.0Zn-14.4Ca in the alloy Zn17Ca13 are a=0.984 nm and c=1.028 nm; but those of the ternary compound with the composition of 44.2Mg-41.3Zn-14.5Ca in the alloy Zn26Ca10 decrease to a=0.951 nm and c=1.007 nm.
In the alloy Zn40Ca10 with three-phase equilibrium at 300 °C (Fig. 3(a)), the composition of the grey phase is 37.2Mg-48.9Zn-13.9Ca. Its hexagonal structure parameters are a=0.945 nm and c=1.003 nm determined by XRD (Fig. 3(b)).
So, the hexagonal structure parameters of the ternary compounds change continuously, i.e. a=0.992-0.945 nm, c=1.034-1.003 nm.
Considering the characteristics of the composition and structure, it could be confirmed that the ternary compound T1 mentioned above is a linear compound. It could be in equilibrium with Mg-based solid solution in the composition range of ~15%Ca, 20.5%-48.9%Zn and balanced Mg. This broad two-phase region (a-Mg+T1) should be helpful for the alloy design.
3.2 Structure of T2 phase and relative phase equilibria
The ternary compounds in the Mg-Zn-Ca alloys reported before are almost in the composition range of T1 phase [16-19]. Besides the black Mg solid solution and grey phase T1, a white phase with the composition of 28.4Mg-63.2Zn-8.4Ca exists in the alloy Zn40Ca10 after equilibrium treatment at 300 °C (Fig. 3(a)). It should be another ternary compound, so-called T2. SAED pattern of the [ ] zone axis of T2 phase is shown in Fig. 3(c). It suggests that T2 phase also has hexagonal structure. The hexagonal structure parameters determined by XRD analysis are a=1.475 nm and c=0.879 nm (Fig. 3(b)).
] zone axis of T2 phase is shown in Fig. 3(c). It suggests that T2 phase also has hexagonal structure. The hexagonal structure parameters determined by XRD analysis are a=1.475 nm and c=0.879 nm (Fig. 3(b)).
According to the morphology (Fig. 3(a)), it can be deduced that T2 phase could form through the peritectic reaction with T1 phase. It has been confirmed further that two ternary compounds in equilibrium with Mg-based solid solution exist in Mg-rich side of Mg-Zn-Ca system. The melting temperature of T1 phase is higher than that of T2 phase. T1 phase is the pre-formed phase during solidification from liquid.

Fig. 2 Microstructures and corresponding XRD patterns of alloys Zn17Ca13 (a, b), Zn32Ca15 (c, d), Zn26Ca10 (e, f) after equilibrium treatment at 300 °C
The equilibrium microstructures and corresponding XRD patterns of the alloys Zn45Ca5 and Zn62Ca5 at 300 °C are shown in Fig. 4 and Fig. 5. In the alloy Zn45Ca5 (Fig4. (a)), the composition of the black phase is 96.2Mg-3.7Zn-0.1Ca, the composition of the grey phase is 50.5Mg-48.0Zn-1.5Ca, and that of the white phase is 26.4Mg-65.5Zn-8.1Ca. It has been confirmed by the XRD analysis (Fig. 4(b)) that the black phase is Mg solid solution, the grey phase is MgZn and the white one is T2 phase.
In the alloy Zn62Ca5 (Figs. 5(a) and (b)), the black phase is MgZn with the composition of 48.2Mg- 50.5Zn-1.3Ca, the grey phase is Mg2Zn3 with the composition of 37.4Mg-61.8Zn-0.8Ca, and the white one is T2 phase with the composition of 27.6Mg-65.3Zn- 7.1Ca.
It could be seen that besides the Mg solid solution and T1 phase, T2 phase could be in equilibrium with MgZn and Mg2Zn3. The composition range of T2 phase is relatively narrow.
3.3 Partial isothermal section of phase diagram of Mg-Zn-Ca system at 300 °C
The partial isothermal section of phase diagram of Mg-rich side in Mg-Zn-Ca system at 300 °C has been constructed according to the above experimental data and is shown in Fig. 6.

Fig. 3 Equilibrium microstructure of alloy Zn40Ca10 held at 300 °C (a), corresponding XRD pattern (b) and SAED pattern of [ ] zone axis of T2 phase (c)
] zone axis of T2 phase (c)
It is shown that the maximum solubility of Zn in Mg solid solution is 3.7% in the Mg-Zn-Ca ternary system at 300 °C, whereas the maximum solubility of Zn is 2.4% in Mg-Zn binary system [1]. The maximum solubility of Ca in the Mg solid solution at 300 °C is relatively low, but the addition of Ca increases the solubility of Zn in Mg-based solid solution, which should affect the solid solution strengthening of the element Zn in the Mg matrix.

Fig. 4 Microstructure (a) and XRD pattern (b) of alloy Zn45Ca5 after equilibrium treatment at 300 °C

Fig. 5 Microstructure (a) and XRD pattern (b) of alloy Zn62Ca5 after equilibrium treatment at 300 °C

Fig. 6 Isothermal section of Mg-rich side in Mg-Zn-Ca ternary system at 300 °C
1.4% Zn can dissolve in the binary compound Mg2Ca, and the solubility of Ca in the MgZn phase is 1.5%.
When the Zn to Ca atomic ratio is between 1.1 and 3.1, T1 phase could be in equilibrium with Mg-based solid solution. The Ca2Mg6Zn3 and Ca2Mg5Zn5 [11] should correspond to one composition in the range of T1 phase, separately.
When the Zn to Ca atomic ratio is larger than 3.1, T2 phase can form in Mg-based solid solution. Because of more Zn addition needed, T2 phase is seldom found in Mg-Zn-Ca alloys. Maybe this is the reason why the existence of T2 phase is doubtful. It is easy for T1 phase to form in the Mg-based alloys because of its broad composition range. But, the T1 phase with different compositions can not be judged correctly, without knowing about the characteristics of the linear compound T1.
The two-phase field composed of T1 and a-Mg is broad and the two-phase field (T2+a-Mg) is narrow. The three-phase fields consisting of a-Mg+Mg2Ca+T1, a-Mg+T1+T2, a-Mg+T2+MgZn and T2+MgZn+Mg2Zn3 exist in Mg-rich side of Mg-Zn-Ca system at 300 °C. It can provide more possibilities for the strengthening phase design.
4 Conclusions
1) There exist two ternary compounds T1 and T2 in equilibrium with Mg-based solid solution in Mg-rich side of the Mg-Zn-Ca system at 300 °C. T1 phase is a linear compound with hexagonal structure. Its structure parameters decrease with the increase of Zn content. The compositon range of T2 phase is narrrow. T2 phase has also hexagonal structure and the parameters are a=1.475 nm and c=0.879 nm.
2) The solubility of Zn increases to 3.7% for the ternary addition of Ca in Mg-based solid solution, although the solubility of Ca in Mg-based solid solution is relatively low.
3) The three-phase fields consisting of a-Mg+ Mg2Ca+T1, a-Mg+T1+T2, a-Mg+T2+MgZn and MgZn+ T2+Mg2Zn3 exist in Mg-rich side of Mg-Zn-Ca system at 300 °C.
References
[1] MASSALSKI T B, OKAMOTO H, SUBRAMANIAN P R, KACPRZAK L. Binary alloy phase diagrams [M]. 2nd ed. Metals Park, Ohio: ASM International, 1996.
[2] MAENG D Y, KIM T S, LEE J H, HONG S J, SEO S K, CHUN B S. Microstructure and strength of rapidly solidified and extruded Mg-Zn alloys [J]. Scripta Mater, 2000, 43: 385-389.
[3] WANG Xiao-liang, LI Chang-rong, GUO Cui-ping, DU Zhen-min, HE Wei. Precipitation behavior of GP zones during ageing process of Mg-Zn alloy[J]. Acta Metallurgica Sinica, 2010, 46(5): 575-580 (in Chinese).
[4] YOU B S, PARK W W, CHUNG I S. The effect of calcium additions on the oxidation behavior in magnesium alloys [J]. Scripta Mater, 2000, 42: 1089-1094.
[5] VOSTRY P, STULIKOVA I, SMOLA B, RIEHEMANN W, MORDIKE B L. Structure and stability of microcrystalline Mg-Ca alloy [J]. Materials Science and Engineering A, 1991, 137: 87-92.
[6] OH J C, OHKUBO T, MUKAI T, HONO K. TEM and 3DAP characterization of an age-hardened Mg-Ca-Zn alloy [J]. Scripta Mater, 2005, 53: 675-679.
[7] NIE J F, MUDDLE B C. Precipitation hardening of Mg-Ca(-Zn) alloys [J]. Scripta Mater, 1997, 37: 1475-1481.
[8] BETTLES C J, GIBSON M A, VENKATESAN K. Enhanced age-hardening behaviour in Mg-4 wt.% Zn micro-alloyed with Ca [J]. Scripta Mater, 2004, 51: 193-197.
[9] GENG L, ZHANG B P, LI A B, DONG C C. Microstructure and mechanical properties of Mg-4.0Zn-0.5Ca alloy [J]. Mater Lett, 2009, 63: 557-559.
[10] WAN Xiao-feng, SUN Yang-shan, XUE Feng, BAI Jing, TAO Wei-jian. Effects of calcium addition on high temperature creep resistance of magnesium alloys with high zinc [J]. Rare Metal Materials and Engineering, 2010, 39(12): 2112-2116 (in Chinese).
[11] VILLARS P, PRINCE A, OKAMOTO H. Handbook of ternary alloy phase diagrams [M]. Metals Park, Ohio: ASM International, 1997.
[12] BRUBAKER C O, LIU Z K. A computational thermodynamic model of the Ca-Mg-Zn system [J]. J Alloys Compd, 2004, 370: 114-122.
[13] WASIUR-RAHMAN S, MEDRAJ M. Critical assessment and thermodynamic modeling of the binary Mg-Zn, Ca-Zn and ternary Mg-Ca-Zn systems [J]. Intermetallics, 2009, 17: 847-864.
[14] LEVI G, AVRAHAM S, ZILBEROV A, BAMBERGER M. Solidification, solution treatment and age hardening of a Mg-1.6wt.%Ca-3.2wt.%Zn alloy [J]. Acta Mater, 2006, 54: 523-530.
[15] ZHANG Y N, KEVORKOV D, LI J, ESSADIQI E, MEDRAJ M. Determination of the solubility range and crystal structure of the Mg-rich ternary compound in the Ca-Mg-Zn system [J]. Intermetallics, 2010, 18: 2404-2411.
[16] JARDIM P M, SOLORZANO G, VANDER SANDE J B. Second phase formation in melt-spun Mg-Ca-Zn alloys [J]. Materials Science and Engineering A, 2004, 381: 196-205.
[17] LARIONOVA T V, PARK W W, YOU B S. A ternary phase observed in rapidly solidified Mg-Ca-Zn alloys[J]. Scripta Mater, 2001, 45: 7-12.
[18] OH-ISHI K, WATANABE R, MENDIS C L, HONO K. Age-hardening response of Mg-0.3at.%Ca alloys with different Zn contents [J]. Materials Science and Engineering A, 2009, 526: 177-184.
[19] ZHOU Tao, CHEN Ding, CHEN Zhen-hua. Microstructures and properties of rapidly solidified Mg-Zn-Ca alloys [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(s1): s101-s106.
Mg-Zn-Ca系富Mg侧三元化合物及
其300 ℃固态相平衡
李洪晓, 任玉平, 马倩倩, 蒋 敏, 秦高梧
东北大学 材料各向异性与织构教育部重点实验室,沈阳110819
摘 要:采用合金平衡组织结构分析法,利用扫描电镜组织观察、电子探针定量成分分析以及X射线衍射和透射电子衍射结构分析,对Mg-Zn-Ca系富Mg区域300 °C的相平衡关系及平衡相成分进行研究。结果表明:300 °C时,2个三元化合物T1和T2都可与Mg基固溶体相平衡。T1相是一个线性化合物相,成分为15% Ca(摩尔分数),20.5%~48.9% Zn,余量为Mg。T1相为六方晶体结构,晶格常数为:a=0.992~0.945 nm, c=1.034~1.003 nm,随T1相中Zn含量的增加而减少。化合物T2相也是六方结构,成分为26.4%~28.4%Mg,63.2%~65.5%Zn以及7.1%~8.4%Ca。300 °C时,Zn在Mg基固溶体中的溶解度随Ca的加入而增大,最大溶解度达到3.7%。在Mg-Zn-Ca系中300 °C等温截面相图的富Mg区域存在着三相区a-Mg+Mg2Ca+T1, a-Mg+T1+T2, a-Mg+T2+MgZn 和 MgZn+T2+ Mg2Zn3。
关键词:Mg-Zn-Ca系;三元化合物;结构;成分;相平衡
(Edited by YANG Hua)
Foundation item: Project (50731002) supported by the National Natural Science Foundation of China; Project (20082030) supported by the Natural Science Foundation of Liaoning Province, China
Corresponding author: LI Hong-xiao; Tel: +86-24-83681676; E-mail: lihx@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)60987-4