极端嗜酸微生物纯培养过程中铁元素的转化
来源期刊:中国有色金属学报(英文版)2017年第5期
论文作者:方京华 刘咏 何万里 覃文庆 邱冠周 王军
文章页码:1150 - 1155
关键词:黄钾铁矾;胞外聚合物;生物冶金;含铁纳米颗粒
Key words:jarosite; extracellular polymer substance; bioleaching; ferruginous nano-particles
摘 要:在培养浸矿微生物过程中,培养基中的铁含量不断减少,一般认为主要是生成了黄钾铁矾沉淀与胞外多聚物。对三种嗜酸浸矿菌Ferroplasma thermophilum, Leptospirillum ferriphilum 和Acidithioobacillus ferrooxidans进行培养,发现他们能在嗜酸环境下生存,而且平均每个细胞生成超过10个纳米颗粒。通过分析纳米颗粒的形态与成分,发现颗粒中含有铁,而且纳米颗粒的产量很高。结果表明,在纯培养过程中减少的铁不仅参与生成黄钾铁矾,同时也被吸收进入细胞,合成含铁的纳米颗粒。
Abstract: Jarosite and extracellular polymer substance generated during pure culture and bioleaching process have been widely accepted the main transformation of decreasing iron in the medium. In the present work, acidophilus bioleaching organisms Ferroplasma thermophilum, Leptospirillum ferriphilum and Acidithioobacillus ferrooxidans were cultured. It was found that they can live in low pH environment, and more than 10 particles in each cell intracellular nano-particles are synthesized in the cells. By analyzing the morphology and chemical composition of nano-particles, they were found to contain iron, and the three microorganisms belonged to high-yielding strains. The results show that the transformation of the decreasing iron ions is not only generating jarosite, but also taken into cells and synthesizing ferruginous nano-particles.
Trans. Nonferrous Met. Soc. China 27(2017) 1150-1155
Jing-hua FANG1, Yong LIU1, Wan-li HE1,2,3, Wen-qing QIN2,3, Guan-zhou QIU2,3, Jun WANG1,2,3
1. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
2. School of Minerals Processing & Bioengineering, Central South University, Changsha 410083, China;
3. Key Laboratory of Biohydrometallurgy, Ministry of Education, Central South University, Changsha 410083, China
Received 8 January 2016; accepted 21 June 2016
Abstract: Jarosite and extracellular polymer substance generated during pure culture and bioleaching process have been widely accepted the main transformation of decreasing iron in the medium. In the present work, acidophilus bioleaching organisms Ferroplasma thermophilum, Leptospirillum ferriphilum and Acidithioobacillus ferrooxidans were cultured. It was found that they can live in low pH environment, and more than 10 particles in each cell intracellular nano-particles are synthesized in the cells. By analyzing the morphology and chemical composition of nano-particles, they were found to contain iron, and the three microorganisms belonged to high-yielding strains. The results show that the transformation of the decreasing iron ions is not only generating jarosite, but also taken into cells and synthesizing ferruginous nano-particles.
Key words: jarosite; extracellular polymer substance; bioleaching; ferruginous nano-particles
1 Introduction
Acidithioobacillus ferrooxidans is a kind of acidophilus, autotrophic, obligate aerobic and gram-negative bacterium that thrives optimally at 30 °C and pH 2 [1-4]. Leptospirillum ferriphilum is gram-negative bacteria, which can grow in extremely acidic environment and grow optimally in inorganic media within pH range of 1.3-2.0 [5,6]. Ferroplasma thermophilum is a kind of acidophilus archaea that thrives optimally at 45 °C and pH 1.0 [7,8]. All those three kinds of microorganisms have the ability of oxidation of ferrous ions and can commonly grow in 9K medium [9].
Jarosite precipitation is a very widespread phenomenon observed in many bacterial cultures, especially in bioleaching process [10]. The jarosites are ammoniojarosites with the formula MFe3(SO4)2(OH)6, where M=K+, Na+, NH4+, Ag+, or H3O+. The formation of jarosite precipitation is a reaction in competition with the hydrolysis reaction giving products of basic ferric hydroxysulphates. In the cultures process of Ferroplasma thermophilum, Leptospirillum ferriphilum and Acidithioobacillus ferrooxidan, the medium is 9K which contains a high concentration of NH4+ ions [9], and the jarosites produced are ammoniojarosites with formula NH4Fe3(SO4)2(OH)6. Abundant iron ions will reduce and form jarosites in the medium.
Extracellular polymer substance (EPS) is also a very widespread phenomenon observed in Acidithiobacillus and Leptospirillum cultures [11,12]. The extracellular polymeric substances of both species mainly consist of neutral sugars and lipids [13]. The functions of the exopolymers seem to mediate attachment to a (metal) sulfide surface, and to concentrate iron ions by complexation through uronic acids or other residues at the mineral surface, thus allowing an oxidative attack on the sulfide. In addition, it also contains iron ions [13,14].
Small-angle X-ray scattering [15,16] is well designed to study the nanoscale structures in a material, which is especially appropriate to measure nanoparticles because of their nanoscale sizes. In addition, nano- particles are also the focus research object to seek after nucleation mechanism and growth manner of materials. LI et al [15] studied the use of the small-angle X-ray scattering technique to probe the nucleation and growth regularity of nanomaterials. WANG et al [17] studied the size and shape evolution of gold nanoparticles in aqueous solution by using real-time small-angle X-ray scattering and ultraviolet-visible spectra at the 1W2A small-angle X-ray scattering station of Beijing synchrotron Radiation Facility, China.
Organisms have evolved complex strategies to maintain optimal intracellular iron pools without compromising cell viability, which include iron- dependent gene regulation using an array of transcriptional regulators from Fur, DtxR and RirA families, iron acquisition in either free form (e.g. Fe21/Mn21 NRAMP family transporters) or sequestered by small molecules (siderophores), intracellular storage proteins such as ferritins, and readily inducible defenses against free-radical formation [15,16].
In the present work, the transformation of decreasing iron in the medium was investigated. The intracellular nano-particles were observed by transmission electron microscope (TEM), the chemical composition of nano-particles was analyzed by energy spectrum analysis, and the grain size of nano-particles was analyzed by small angle X-ray scattering.
2 Materials and methods
A. ferrooxidans f1, F. thermophilum L1T (EF062309) and L. ferriphilum YSK used in this work were provided by the Key Laboratory of Biohydrometallurgy, Ministry of Education, China. The strains were cultivated in basal salts of modified 9K medium, the pH of A. ferrooxidans f1 was adjusted to 2.0, by adding 50% H2SO4 (volume fraction), and the culture temperature was 30 °C; the pH of F. thermophilum L1T was adjusted to 1.0, and the culture temperature was 45 °C; the pH of L. ferriphilum YSK was adjusted to 1.6, and the culture temperature was 45 °C. FeSO4·7H2O was added into the medium, after that these microorganisms were cultured at rotation speed of 170 r/min. Additional 0.2% yeast extract was added to F. thermophilum L1T.
Ultrathin sections were obtained with an ultramicrotome (LKB-5, LKB, Sweden) and then stained with uranyl acetate and lead citrate. The morphologies were observed by TEM (FEI Tecnai Spirit) at an accelerating voltage of 80 kV.
A drop of cell was deposited onto a copper grid (62 μm) covered by a carboncoated formvar film. The cells were allowed to settle on the grid for 10 min, and excess liquid was removed with a filter paper. All grids were rinsed at least twice with distilled water before TEM observation. In case of negative staining, wet grids were treated with a drop of negative staining solution (1.5% uranyl acetate) for 1 min and then observed by TEM (JEM2100F) at an accelerating voltage of 200 kV.
The concentrations of total iron in solution were measured by atomic absorption spectrometry, the ferrous iron concentration was determined by titration with potassium dichromate and the ferric iron concentration was equal to the difference between the concentrations of total iron and ferrous iron. The grain size of nano-particles was analyzed by small angle X-ray scattering at the small angle scattering experiment station (1W2A) at Beijing Synchrotron Radiation Facility, China.
3 Results and discussion
3.1 Morphology of nano-particles
TEM observation was used to analyze the morphology inside the microorganisms. As shown in Fig. 1, nano-particles are seen in A. ferrooxidans f1, F. thermophilum L1T and L. ferriphilum YSK. The arrangements of the nano-particles inside the three strains are different, the arrangement of the nano- particles is random and disperse in the A. ferrooxidans f1 and L. ferriphilum YSK. However, in F. thermophilum L1T, the nano-particles are gathered. The size of the nano-particles is inhomogenous, and there are more than 10 nano-particles in each cell.

Fig. 1 TEM images of microorganisms
It has been reported that magnetotactic bacteria (MTB) [18-20], living in the anaerobic or microaerobic neutral or alkaline environment [21-23], biomineralize unique organelles called magnetosomes which are usually arranged as a chain within the cell, thereby maximizing the magnetic dipole moment of the cell and causing the cell to passively align along magnetic field lines as they swim [24]. Magnetosomes consist of magnetic mineral crystals, either magnetite (Fe3O4) or greigite (Fe3S4), enveloped by a bilayer membrane composed mostly of phospholipids, called the magnetosome membranes, which contain a number of proteins not present in the cytoplasmic and outer membranes (OMs) and are unique to MTB [19]. Obviously, there are differences between the nanocrystals and magnetosomes. TEM images can prove magnetosomes are membrane-enveloped. However, for the nanoparticles, there is no direct evidence demonstrating the nanocrystals are membrane-enveloped. Normally, magnetosomes are organized into one or multiple chain-like structures within the cell in order to optimize the cellular magnetic dipole moment. It is more like a compass needle to facilitate the navigation of MTB using the Earth’s magnetic field, which causes MTB respond and orient along the lines of magnetic fields to find the anaerobic or microaerobic environment for living [24]. However, the arrangement of nanocrystals in A. ferrooxidans f1, F. thermophilum L1T and L. ferriphilum YSK is irregular, not in a line, and it may get together, as shown in Fig. 2(a). Take F. thermophilum for example, the nano-particles seem globular (Fig. 2(b)) and tooth-shaped (Fig. 2(c)), respectively. The size of magnetosomes is generally 20-30 nm, while the size of the nano-particles is inhomogenous, the size of nano-particles of A. ferrooxidans f1 is 35-45 nm; for F. thermophilum L1T, the size of nano-particles is approximately 20-30 nm; for L. ferriphilum YSK, the size of nano-particles approximately is 40-50 nm, as shown in Fig. 3. It has previously reported that Acidithiobacillus ferrooxidans can produce magnetite, and the number of those magnetie is 1-3 per cell [25,26]. In this work, more than 10 nano-particles are found in A. ferrooxidans f1, F. thermophilum L1T, L. ferriphilum YSK in each cell. It is suggested that A. ferrooxidans f1, F. thermophilum L1T and L. ferriphilum YSK not only can synthesize intracellular nanometre-sized particles, but also form a kind of high-yield nano-particle producing strains.

Fig. 2 TEM images (a, b, c) of microorganisms in F. thermophilum and corresponding EDX spectra (d)
3.2 Chemical composition of nano-particles
There are plenty of nano-particles in the cell, however, the morphology of nano-particles is not the same. The energy dispersive X-ray (EDX) analysis results show that the nano-particles contain iron. Hence, it can be inferred that the transformation of the decreasing iron in the medium not only form jarosite and EPS, but also form intracellular ferruginous-nano- particles.
However, the chemical composition of the nano-particles is different from that of magnetosomes which contains iron minerals of magnetite (Fe3O4) and/or greigite (Fe3S4) and high amount of iron, oxygen and/or sulfur [19,20]. As shown in Fig. 2(d), the nano-particles contain iron, cobalt and oxygen, but no sulphur. It is found that the nano-particles of A. ferrooxidans f1, F. thermophilum L1T and L. ferriphilum YSK contain cobalt as well in nature conditions. It is notable that no cobaltic ions are directly added into the culture medium, the source of cobalt is from the FeSO4·7H2O that was added into the medium, which is not pure and mixed with cobalt ions, as indicated by ICP-OES analysis.
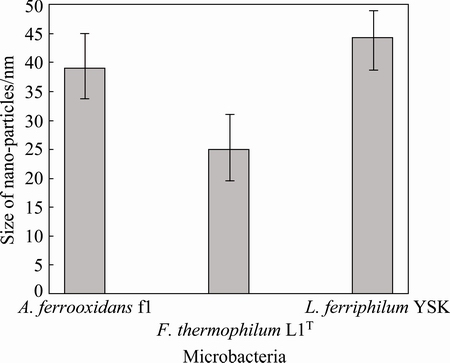
Fig. 3 Size of nano-particles of three microorganisms obtained by small angle X-ray scattering
3.3 Transformation of decreasing iron in medium
In the bioleaching process, the concentration of total iron decreases after an initial increase, as shown in Fig. 4. The reason of this phenomenon is that the iron is dissolved step by step from minerals containing iron, such as copper pyrites and bornite, hence, there is an initial increase. However, with the formation of jarosite and other ferruginous substance, the concentration of iron decreases. In the pure culture process, the concentration of total iron constantly decreases, as shown in Fig. 5. By the biological oxidation of microorganisms, the ferrous irons are constantly transformed to ferric irons, hence, the ferrous irons constantly decrease, and the ferric irons increase. Because the energy source is FeSO4·7H2O, it immediately dissolves once added into medium, so it does not have an initial increasing stage. Anyway, the total iron decreases both in bioleaching and pure culture process. Previous research suggested that the transformation of the decreasing iron ions in the medium is forming ferruginous sediment and uptaken by cells [27,28]. As iron is an essential nutrient, the cell uptakes it into cystolic, it is clear that microorganisms thrive in acidic ecosystems with high iron loads requiring a comprehensive investigation of the strategies to acquire iron and to coordinate this acquisition with utilization, storage and oxidation of iron through metal responsive regulation, however, the content of iron is very low, hence, the main transformation of the decreasing iron ions in the medium is forming jarosite and EPS. In this work, the decreasing iron ions in the medium are also uptaken by cell and form abundant ferruginous nano-particles, as shown in Fig. 6. By the interaction of biological oxidation, Fe2+ is transferred to Fe3+. After a pure culture for a long time, part of them would transfer to jarosite, and the cell would uptake Fe2+ and Fe3+ to form EPS.

Fig. 4 Total iron concentration in bioleaching process
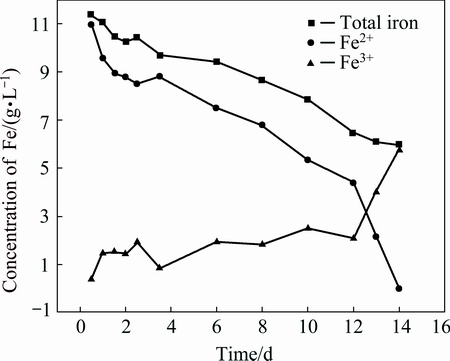
Fig. 5 Iron concentration in pure culture process
3.4 Cycle of iron in extreme acid environment
In the nature extreme acid environment, the main valence states of iron are Fe2+ and Fe3+, which can interconvert, and the Fe2+ is oxidized to Fe2+ by biological or chemical oxidation. L. ferriphilum YSK, A. ferrooxidans f1 and F. thermophilum L1T have enzymes of iron (II) ion oxidation, so they have the ability to oxide Fe2+ to Fe3+ and also the Fe2+ can be reduced to Fe3+ by biological and chemical reduction [1,5,8]. Meanwhile, the cell will uptake iron ions both Fe2+ and Fe3+ into cystolic by ion channel. Acidithiobacilli and the Leptospirilla have been predicted FeoB-like Fe (II) and Nramp-like Fe(II)-Mn(II) transporters exhibiting a plethora of predicted TonB-dependent Fe(III) transport systems [29]. It will accumulate within the cells during the formation of intracellular ferruginous nano-particles, however the biochemical/chemical pathways of which are still not completely understood. When cells die, parts of or entire magnetofossils could be dissolved thereby releasing Fe2+ and Fe3+ ions back to environmental iron cycling, as shown in Fig. 7. While other magnetofossils may be deposited into sediments eventually leading to mineral iron formation.
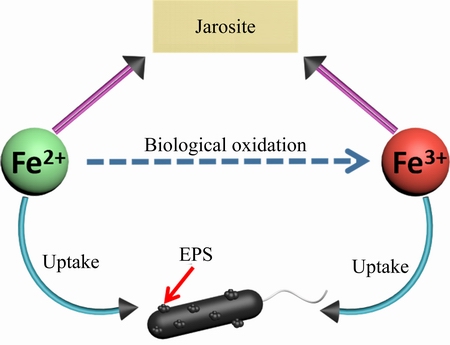
Fig. 6 Schematic diagram showing bioleaching process
Up to now, it is just discovered that three kinds of extreme acid microorganisms can synthesize ferruginous nano-particles, the species involve bacteria and archaea, and the morphology involves rhabditiform, heliciform and globular, which may be a universal function of extreme acid microorganisms. More research involving the distribution and ecology of extreme acid microorganisms that can synthesize ferruginous nano-particles is necessary, which helps to better understand the conditions under which and to what extent extreme acid microorganisms affect the biogeochemical cycle of iron. As shown in Fig. 7, by the interaction of biological or chemical oxidation, Fe2+ is transferred to Fe3+; while by the interaction of biological or chemical reduction, Fe3+ is transferred to Fe2+, so the bacteria uptakes both Fe2+ and Fe3+. When the bacteria die, the bacteria transfer to magnerofossils. Then the magnerofossils dissolute into iron biominerals, and they dissolute into Fe2+ and Fe3+, meanwhile the iron biominerals also dissolute into Fe2+ and Fe3+.

Fig. 7 Circle process of iron, bacteria and biominerals
4 Conclusions
1) The transformation of decreasing iron in media is investigated, in the pure culture process, the total iron constantly decreases. While in the bioleaching process, the total iron decreases after an initial increase.
2) Acidithioobacillus ferrooxidans f1, Ferroplasma thermophilum L1T and Leptospirillum ferriphilum YSK have the ability to synthesize intracellular nano-particles containing iron, and the size of nano-particles is inhomogenous. There are more than 10 particles in each cell, so the three microorganisms belong to high-yielding strains.
3) The transformation of iron is not only generating jaosite and EPS, but is also taken into cell and synthesizing ferruginous nano-particles.
References
[1] AMOURIC A, BROCHIER-ARMANET C, JOHNSON D B, BONNEFOY V, HALLBERG K B. Phylogenetic and genetic variation among Fe (II)-oxidizing acidithiobacilli supports the view that these comprise multiple species with different ferrous iron oxidation pathways [J]. Microbiology-SGM, 2011, 157: 111-122.
[2] KELLY D P, WOOD A P. Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov and Thermithiobacillus gen. nov [J]. International Journal of Systematic and Evolutionary Microbiology, 2000, 50: 511-516.
[3] LILJEQVIST M, RZHEPISHEVSKA O I, DOPSON M. Gene identification and substrate regulation provide insights into sulfur sccumulation during bioleaching with the psychrotolerant acidophile Acidithiobacillus ferrivorans [J]. Applied and Environmental Microbiology, 2013, 79: 951-957.
[4] LILJEQVIST M, VALDES J, HOLMES D S, DOPSON M. Draft genome of the psychrotolerant acidophile Acidithiobacillus ferrivorans SS3 [J]. Journal of Bacteriology, 2011, 193: 4304-4305.
[5] CORAM N J, RAWLINGS D E. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptosphillum ferriphilum sp nov dominates South African commercial biooxidation tanks that operate at 40 °C [J]. Applied and Environmental Microbiology, 2002, 68: 838-845.
[6] LIU J S, XIE X H, XIAO S M, WANG X M, ZHAO W J, TIAN Z L. Isolation of Leptospirillum ferriphilum by single-layered solid medium [J]. Journal of Central South University of Technology, 2007, 14: 467-473.
[7] ZHANG L, ZHOU W, LI K, MAO F, WAN L, CHEN X, ZHOU H, QIU G. Synergetic effects of Ferroplasma thermophilum in enhancement of copper concentrate bioleaching by Acidithiobacillus caldus and Leptospirillum ferriphilum [J]. Biochemical Engineering Journal, 2015, 93: 142-150.
[8] ZHOU H, ZHANG R, HU P, ZENG W, XIE Y, WU C, QIU G. Isolation and characterization of Ferroplasma thermophilum sp nov., a novel extremely acidophilic, moderately thermophilic archaeon and its role in bioleaching of chalcopyrite [J]. Journal of Applied Microbiology, 2008, 105: 591-601.
[9] SILVERMAN M P, LUNDGREN D G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. II. Manometric studies [J]. Journal of Bacteriology, 1959, 78: 326-331.
[10] DAOUD J, KARAMANEV D. Formation of jarosite during Fe2+ oxidation by Acidithiobacillus ferrooxidans [J]. Minerals Engineering, 2006, 19: 960-967.
[11] CRUNDWELL F K. How do bacteria interact with minerals? [J]. Hydrometallurgy, 2003, 71: 75-81.
[12] VERA M, SCHIPPERS A, SAND W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—Part A [J]. Applied Microbiology and Biotechnology, 2013, 97: 7529-7541.
[13] SAND W, GEHRKE T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron (III) ions and acidophilic bacteria [J]. Research in Microbiology, 2006, 157: 49-56.
[14] JENSEN A B W C. Ferrous sulphate oxidation using ThiobaciZZus ferrooxidans: A review [J]. Process Biochemistry (Oxford), 1995, 30 (3): 225-236.
[15] LI Z Z, WU Z H, MO G, XING X Q, LIU P. A small-angle X-ray scattering station at Beijing Synchrotron Radiation Facility [J]. Instrumentation Science & Technology, 2014. 42(2): 128-141.
[16] LI Z Z. A program for SAXS data processing and analysis [J]. Chinese Physics C, 2013, 37(10): 110-115.
[17] WANG W, ZHANG K, CAI Q, MO G, XING X Q, CHENG W D, CHEN Z J, WU Z H. Real-time SAXS and ultraviolet-visible spectral studies on size and shape evolution of gold nanoparticles in aqueous solution [J]. European Physical Journal B, 2010, 76(2): 301-307.
[18] KOMEILI A. Molecular mechanisms of compartmentalization and biomineralization in magnetotactic bacteria [J]. Fems Microbiology Reviews, 2012, 36: 232-255.
[19] FAIVRE D, SCHUELER D. Magnetotactic bacteria and Magnetosomes [J]. Chemical Reviews, 2008, 108: 4875-4898.
[20] UEBE R, SCHUELER D. Magnetosome biogenesis in magnetotactic bacteria [J]. Nature Reviews Microbiology, 2016, 14: 621-637.
[21] LEFEVRE C T, BAZYLINSKI D A. Ecology, diversity, and evolution of magnetotactic bacteria [J]. Microbiology and Molecular Biology Reviews, 2013, 77: 497-526.
[22] BAZYLINSKI D A, LEFEVRE C T. Magnetotactic bacteria from extreme environments [J]. Life, 2013, 3: 295-307.
[23] LEFEVRE C T, FRANKEL R B, POSFAI M, PROZOROV T, BAZYLINSKI D A. Isolation of obligately alkaliphilic magnetotactic bacteria from extremely alkaline environments [J]. Environmental Microbiology, 2011, 13: 2342-2350.
[24] LIN W. BAZYLINSKI D A, XIAO T, WU L, PAN Y. Life with compass: Diversity and biogeography of magnetotactic bacteria [J]. Environmental Microbiology, 2014, 16: 2646-2658.
[25] YAN L, YUE X, ZHANG S, CHEN P, XU Z, LI Y, LI H. Biocompatibility evaluation of magnetosomes formed by Acidithiobacillus ferrooxidans [J]. Materials Science & Engineering C, 2012, 32: 1802-1807.
[26] YAN L, ZHANG S, CHEN P, WANG W, WANG Y, LI H. Magnetic properties of Acidithiobacillus ferrooxidans [J]. Materials Science & Engineering C, 2013, 33: 4026-4031.
[27] POTRYKUS J, JONNA V R, DOPSON M. Iron homeostasis and responses to iron limitation in extreme acidophiles from the Ferroplasma genus [J]. Proteomics, 2011, 11: 52-63.
[28] WEAVER E A, WYCKOFF E E, MEY A R, MORRISON R, PAYNE S M. FeoA and FeoC are essential components of the vibrio cholerae ferrous iron uptake system, and FeoC interacts with FeoB [J]. Journal of Bacterinology, 2013, 195: 4826-4835.
[29] OSORIO H, MARTINEZ V, NIETO P A, HOLMES D S, QUATRINI R. Microbial iron management mechanisms in extremely acidic environments: Comparative genomics evidence for diversity and versatility [J]. BMC Microbiology, 2008, 8(1): 1-18.
方京华1,刘 咏1,何万里1,2,3,覃文庆2,3,邱冠周2,3,王 军1,2,3
1. 中南大学 粉末冶金国家重点实验室,长沙410083;
2. 中南大学 资源加工与生物工程学院,长沙410083;
3. 中南大学 生物冶金教育部重点实验室,长沙410083
摘 要:在培养浸矿微生物过程中,培养基中的铁含量不断减少,一般认为主要是生成了黄钾铁矾沉淀与胞外多聚物。对三种嗜酸浸矿菌Ferroplasma thermophilum, Leptospirillum ferriphilum 和Acidithioobacillus ferrooxidans进行培养,发现他们能在嗜酸环境下生存,而且平均每个细胞生成超过10个纳米颗粒。通过分析纳米颗粒的形态与成分,发现颗粒中含有铁,而且纳米颗粒的产量很高。结果表明,在纯培养过程中减少的铁不仅参与生成黄钾铁矾,同时也被吸收进入细胞,合成含铁的纳米颗粒。
关键词:黄钾铁矾;胞外聚合物;生物冶金;含铁纳米颗粒
(Edited by Sai-qian YUAN)
Foundation item: Project (51374248) supported by the National Natural Science Foundation of China; Project (NCET-13-0595) supported by the Program for New Century Excellent Talents in University of China; Project (2016-SSRF-PT-006152) supported by the Shanghai Synchrotron Radiation Facility (SSRF), China; Project (2016-BEPC-PT-000855) supported by the Beijing Synchrotron Radiation Facility (BSRF), China
Corresponding author: Wan-li HE; Tel: +86-15274946963; E-mail: 1281108420@qq.com
DOI: 10.1016/S1003-6326(17)60134-1

