Photocatalytic properties of TiO2 bonded active carbon composites prepared by SOL-GEL
LI You-ji(李佑稷)1, 2, LI Xiao-dong(李效东)2, LI Jun-wen(李君文)3, YIN Jing(尹 静)3
(1. College of Chemistry and Chemical Engineering, Jishou University, Jishou 416000, China;
2. State Key Laboratory of New Ceramic Fibers and Composites,School of Aerospace and Materials Engineering,National University of Defence Technology, Changsha 410073, China;
3. Institute of Hygiene and Environmental Medicine, Academy of Military Medical Science, Tianjin 300050, China)
Abstract: Photocatalyst of TiO2 bonded active carbon (TiO2/AC), was prepared via sol-gel method from a mixture of TiO2 sol with active carbon. Post heat treatment was performed at 250℃ for 2h in air and then kept at 400℃ to 600℃ under a flow of nitrogen for 2h. The TiO2/AC composites obtained were characterized by SEM, XRD, UV-vis and BET. The photocatalytic activities of the TiO2/AC composites were studied in comparison with TiO2, AC, P-25 and a mixture of TiO2 and AC, respectively. The Ramnant rate of Rhodamine B absorbed by the active carbon is found to be almost 70% and the remnant rates of the Rhodamine B decolorized by TiO2 and the mixture of TiO2 and the active carbon are 30% and 25%, respectively. However, nearly complete removal of Rhodamine B is observed for a TiO2/AC composite after 200min under UV irradiation, which will take the P-25 commercial product 5h. Therefore, the TiO2/AC composite is much more effective in decolorization of aqueous Rhodamine B. In addition, the composite can be easily separated from solutions.
Key words: TiO2-bonded composite; sol-gel; photoactivity; active carbon CLC number: TQ134
Document code: A
1 INTRODUCTION
Environmental purification using photocatalysts has attracted a great deal of attention due to the increasing environmental problems in the world[1]. Recently, their application has been focused on the purification and treatment of water and air[2]. TiO2 is the a promising and wildly used photocatalyst because of its high activity, chemical stability, robustness against photocorrosion, low toxicity, no-twain pollution and availability at low cost[3-5]. However, shortcomings of conventional powder catalysts are owing to the low efficiency in making use of light, difficulty in stirring during reaction and separation after reaction and low-concentration contamination near TiO2[6, 7]. These disadvantages of TiO2 result in low efficiency in photocatalytic activity in practical application. Correspondingly, catalysts such as TiO2 bonded active carbon(TiO2/AC) were prepared to overcome these disadvantages and to extend its industrial applications. It is efficient to shelter low-concentration contamination that the higher BET surface area of active carbon act as the carrier of TiO2 powder[8]. Some papers reported on preparation of the composites between TiO2 and carbon[9-12] and TiO2-mounted exfoliated graphite[13, 14]. It was reported that the addition of active carbon to titania slurry could increase decomposition of some organic compounds during the photocatalytic process[15]. In other groups work, mounting of TiO2 onto various active carbons was reported to result in certain reduction of specific surface area of carbons and TiO2 broke away from active carbon[12]. This was reasonably supposed to be owing to the preferential deposition of TiO2 particles at the entrance of the miniature pores of active carbon and TiO2 particles felted on the surface of active carbon by fixed glue . In the present work, the sol-gel technique is applied in order to introduce TiO2 into the inside and outside of active carbon and increase their binding energy, aiming to avoid precipitation of TiO2 into miniature pores of active carbon by adjusting the ratio of active carbon to TiO2 sol and restrict TiO2 desquamating from active carbon by heat treatment. In this work, composites of TiO2/AC with high photocatalytic activity are successfully prepared and their photocatalytic properties are studied.
2 EXPERIMENTAL
2.1 TiO2/AC composites preparation
Precursor solutions for TiO2 /AC were prepared by the method[3] as follows. Tetrabutylorthotitanate(Aldrich, 99.9%, 17.02mL) and diethanoiamine(4.8mL) were dissolved in ethanol(64.82mL). The solution was stirred vigorously for 2h at 20℃ followed by addition of a mixture of distilled water(0.9mL) and ethanol(10mL) on stirring. The resulted alkoxide solution was left standing at 20℃ for 2h for hydrolysis reaction, resulting in the TiO2 sol. The chemical composition of the starting alkoxide solution was x[Ti-(OC4H9)4]∶x(C2H5OH)∶x(H2O)∶x[NH-(C2H4OH)2]=1∶25.5∶1∶1. Then a desired amount of active carbon particles(AC)(pure chemicals)was used as the carriers and was added into TiO2 sol. After this sol changed to gel, TiO2 gel bonded active carbon was heat treated at 250℃ for 2h in air and then heating temperature was increased gradually to the end temperature form 400℃ to 600℃ for 2h in nitrogen using an electric oven. The concentration of TiO2 sol in active carbon was adjusted by the quantity of active carbon added to TiO2 sol.
2.2 Photocatalytic activity evaluation
Photocatalytic activity of the TiO2/AC was determined by aqueous Rhodamine B decolorization in water under UV irradiation. The same mass(0.5g) of TiO2/AC, AC, TiO2 and mixture of TiO2 with AC(the amount of TiO2 in mixture is equal to that of TiO2 in TiO2/AC) were added respectively into aqueous Rhodamine B with a concentration of 5mmol/L in a quartz cell(280mL), as presented in Fig.1. An ultraviolet lamp of 40W, fixed in the middle of the quartz cell, was used as a light source, with wavelength range and peak wavelength of 320-400nm and 365nm, respec-

Fig.1 Setup of photocatalytic reaction
tively. The solution was sparged with air during irradiation. The concentration of Rhodamine B after decolorization was determined by an UV-visible spectrophotometer. The photocatalytic decolorization of Rhodamine B is a pseudo-first-order reaction and its kinetics may be expressed as

2.3 Characterization
The crystallinity of the TiO2/AC was determined by X-ray diffraction(XRD) using an HZG4-PC diffractometer with CuKα radiation, the accelerating voltage and the applied current were 35kV and 20mA, respectively. The crystalline size of TiO2/AC and TiO2 was calculated from XRD measurement by supplied computer program. The concentration of anatase was estimated from integrated intensities of the reflection of(101) and(110) phases. The amount of TiO2 bonded on the carbon surface was determined from ignition loss at 700℃ in air by using TG apparatus(STA 449 C Jupiter thermobalance of Netzsch Company, Germany). The structure of prepared catalysts was observed on using scanning electron microscope(SEM, SX-100) with an accelerating voltage of 20kV. The size of TiO2 grains in TiO2/AC composites and TiO2 was observed using an UV-visible spectrophotometer(Heλiosα of Unicam company, England) with a wavelength range of 200-1000nm. BET surface area was determined with a monosorb BET analyzer(Quantachrome Company, USA).
3 RESULTS AND DISCUSSION
3.1 Photocatalytic activity of TiO2/AC
The results of Rhodamine B removal under UV irradiation are presented in Fig.2. The remnant rate of Rhodamine B, decolorized by active carbon under UV irradiation without photocatalysis, was found to be 70% after 200min. The remnant rate of the Rhodamine B decolorized by the TiO2 and UV irradiation are 30% and 96% after 200min, respectively. However, heated to an end temperature of 500℃ for 2h, the TiO2/AC containing both anatase and rutile phase reaches almost 100% of the Rhodamine B removal. This seems to suggest that the effect of the active carbon carriers must appear remarkably. To demonstrate further the utility of the active carbon carriers for TiO2 loading, photodecomposition of Rhodamine B was studied using the naked TiO2 in the presence of dispersed active carbon. As shown in Fig.2, the reaction rate of the naked TiO2 was increased by introducing the active carbon into the TiO2 suspension, The remnant rate of the Rhodamine B decolorized by the mixture of TiO2 with active carbon was 25%, but the enhancement was not so great as that obtained at the 26.3%-loaded TiO2/AC. It may attribute this to the active carbon with high surface area, which worked well as an effective adsorbate to concentrate Rhodamine B around the loaded TiO2. The adsorbed Rhodamine B seems to be supplied to the loaded TiO2 mostly by surface diffusion. In addition, active carbon can possibly prevent recombination of electron-hole pairs. The presence of Ti3+ ion, which could capture generated electrons, is confirmed by XPS survey spectra under effect of active carbon. UV irradiation was essential to photodegradation of Rhodamine B by TiO2/AC, because the remnant rate of the Rhodamine B decolorized by the TiO2/AC without UV is 68%.
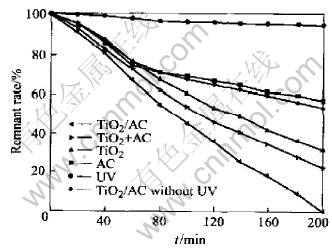
Fig.2 Photocatalytic degradation properties of TiO2/AC, TiO2, AC and mixture of TiO2 powder with AC, pyrolyzed under same condition at 500℃
The photoactivity was compared to commercially available TiO2 P-25(Degussa, Germany) that consists of both anatase(80%) and rutile(20%) phases of TiO2. The same experimental conditions were selected for P-25. From the comparison between catalysts P-25 and the TiO2/AC composite(in Fig.3.), it can be seen that Rhodamine B undergoes decomposition much faster in the case of the latter catalyst. After 200min of irradiation almost 100% Rhodamine B removal was observed, but the former needed at least 5h.
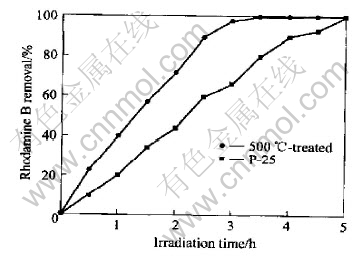
Fig.3 Rhadamine B removal under UV irradiation with P-25 and 500℃-treated catalysts
IR spectra of Rhodamine B show that some organic compounds emerged after it was photodegraded, as illustrated in Fig.4. The new bands in the range of 1150-1270cm-1 are attributed to C-O vibration of carbonate or ether species. The apparent reaction rate constants of the photocatalytic decolorization of Rhodamine B was 0.0077min-1 for TiO2/AC and 0.0050min-1 for TiO2, respectively.

Fig.4 IR spectra of Rhodamine B before(a) and after(b) decolorization
3.2 XRD results
XRD patterns of TiO2/AC and TiO2 under different heat-treated temperatures are presented in Fig.5 and Table 1. The TiO2/AC heated to 450℃ for 2h consists only of anatase phase of TiO2, while that heated at 500℃ consists of both anatase(65%) and rutile(35%). Crystalline phase of anatase was developed in TiO2/AC with the increase of heat-treatment temperature. The higher the heat-treatment temperature, the larger the amount of rutile formed. When the heat-treatment temperature was 550℃ for 2h, TiO2/AC consists of only rutile. However, the TiO2 consists of both anatase(95%) and rutile(5%) phases at heat-treatment temperatre of 450℃ for 2h. When the TiO2 was heated from 500℃ to 550℃, the content of anatase of TiO2 varied from 45% to 0. The crystallite size of TiO2/AC and TiO2 were rising with the increasing heat-treatment temperature. At the same heat-treatment temperature, the crystallite size of TiO2 was greater than that of TiO2/AC and the increasing degree of the grain size of TiO2 is faster than that of TiO2/AC. This may be attributed to the fact that the active carbon has such a great surface area that it retards the growth of TiO2 crystallite bonded active carbon.
3.3 UV-vis spectra
Fig.6 shows the UV-vis spectra in the wave-
Table 1 Anatase content and crystalline grain size of TiO2/AC and TiO2 under different heat-treatment conditions for 2h
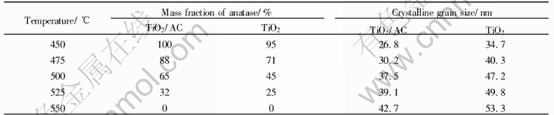
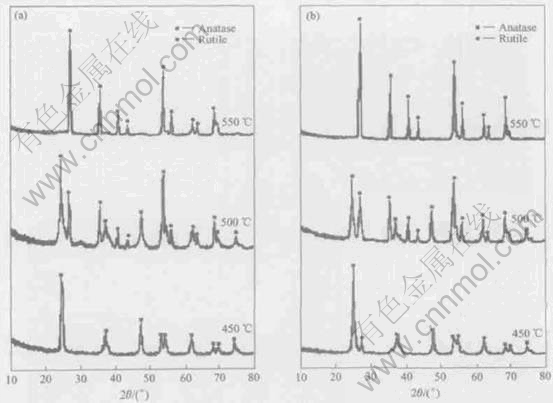
Fig.5 XRD patterns of TiO2/AC composites(a) and TiO2 nano-powders(b) pyrolyzed at different temperatures
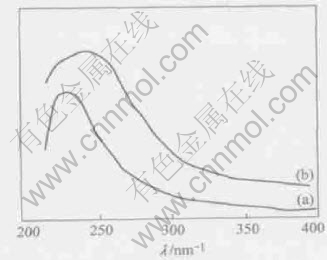
Fig.6 UV-vis absorption spectra of TiO2/AC(a) and TiO2(b)
length range 200-400nm for TiO2 grains in composites and TiO2 grains, respectively. The absorption edge of the TiO2/AC(Fig.6(a)) is observed at a lower wavelength range than that of the TiO2(Fig.6(b)). The shift is ascribed to the difference in particle size. The TiO2/AC composites contain relatively small particle and show a bseudo“blue shift”. This is adapt to the obtained results by XRD. The BET surface areas of TiO2/AC, TiO2 and active carbon are presented in Table 2. The BET surface area of the active carbon in TiO2/AC rises slightly with the increasing heat-treatment temperature. At the same time, the BET surface area of TiO2 reduces as the grain size of TiO2 raised. It causes development of surface area of TiO2/AC that the big carves in active carbon changes into small carves with the decreasing mass of TiO2/AC.
3.4 SEM results
Fig.7 shows the scanning electron micrograph of the surface of TiO2/AC and TiO2. It is observed
Table 2 BET surface area of TiO2/AC and TiO2 under different heat-treatment conditions
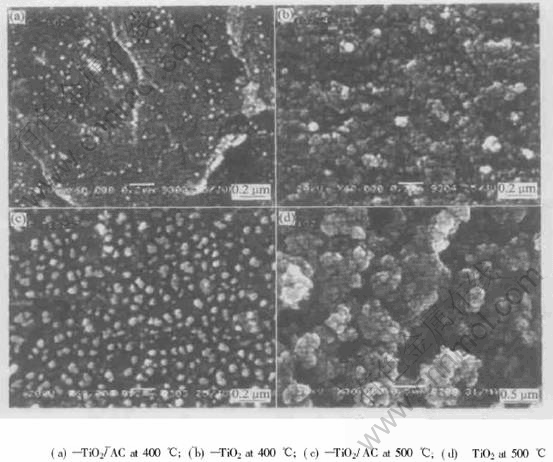
Fig.7 SEM images after heat-treated under different conditions for 4h
that the TiO2/AC has spherical microstructure and dispersing texture, composed of 30-50nm sphere particles. However, the grain size of TiO2 with reuniting microstructure is bigger than that of TiO2/AC. In accordance with the increasing heat-treatment temperature, the grain sizes of TiO2/AC and TiO2 increase, but the increasing degree of the grain size of TiO2 is faster than that of the grain size of TiO2/AC. This is due to the great surface area of the active carbon that has a great absorbing energy” to retard growth of TiO2 grains.
4 CONCLUSIONS
In order to prepare TiO2 bonded active carbon composite with higher photoacativity, the active carbon was added into TiO2 sol by properly controlling the ratio of active carbon to TiO2 sol. Introducing active carbon into the TiO2 composite would not decrease its surface area, instead, some advantages can be obtained, such as suppression of anatase onto rutile phase and retarding growth of TiO2 grains .The prepared catalysts can effectively remove rhodamine B under UV irradiation and can be easily separated from solutions, which is due to the active carbon carrier
REFERENCES
[1]Kawasaki K, Despres J F, Kamei S, et al. Photocatalytic oxidation of organics in water using pure and silver-modified titanium dioxide particles [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2002, 148: 233-245.
[2]Gao W, Wu F Q, Luo Z, et al. Studies on the relationship between the crystal form of TiO2 and its photocatalyzing degradation efficiency [J]. Chem J Chinese Unversities, 2001, 22: 660.
[3]Znaidi L, Seraphimova R, Bocquet J F, et al. A semi-continuous process for the synthesis of nanosize TiO2 powder and their use as photocatalysts [J]. Mat Res Bull, 2001, 36: 812.
[4]Hoffman M R, Martin S T, Choi W. Environmental application of semiconductor photocatalysis [J]. Chem Rev, 1995, 95: 69-96.
[5]Vicente G S, Morales A, Gutierrez M T. Preparation and characterization of sol-gel TiO2 antireflective coatings for silion [J].Thin Solid Films, 2001, 391: 133-137.
[6]Herrmann J M, Guillard C, Disdier J, et al. New industrial titania photocatalysts for the solar detoxification of water containing various pollutants [J]. Applied Catalysis B: Environmental, 2002, 35: 281-294 .
[7]Takeda N, Torimoto T, Sampath S, et al. Effect of inert supports for titanium dioxide loading on enhancement of photodecomposition rate of gaseous propionaldehyde [J]. J Phys Chem, 1995, 99: 9986-9991.
[8]Uchida H, Itoh S, Yoneyama H. Photocatalytic decomposition of propyzamide using TiO2 supported on activated carbon [J]. Chem Lett, 1993, 22: 1995-1998.
[9]El-Sheikh A H, Newman A P, Al-Daffaee H, et al. deposition of anatase on the surface of activated carbon [J]. Surface and Coatings Technology, 2004, 187: 284-292.
[10]Lettmann C, Hildenbrand K, Kisch H, et al. Visible light photodegradation of 4-chlorophenol with a coke-containing titanium dioxide photocatalyst [J]. Appl Catal B, 2001, 32: 215-272.
[11]Zhang F S, Nriagu J O, Itoh H. Photocatalytic removal and recovery of mercury from water using TiO2-modified sewage sludge carbon [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2004, 167: 223-228.
[12]Tryba B, Morawski A W, Inagaki M. A new route for preparation of TiO2-mounted activated carbon [J]. Appl Catal B, 2003, 41: 427.
[13]Tsumura T, Kojitani N, Umemura H, et al. Composites between photoactive anatase-type TiO2 and adsorptive carbon [J]. Appl Surf Sci, 2002, 196: 429-436.
[14]Modestov A.D, Lev O. Photocatalytic oxidation of 2, 4-dichlorophenoxyacetic acid with titania photocatalyst. Comparison of supported and suspended TiO2 [J]. J Photochem Photobiol A, 1998, 112: 261-270.
[15]Silva C G, Faria J L. Photochemical and photocatalytic degradation of an azo dye in aqueous solution by UV irradiation [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2003, 155: 133-143.
(Edited by LONG Huai-zhong)
Foundation item: Project supported by the National Defence of Academy of Military Medical Science
Received date: 2004-03-16; Accepted date: 2004-09-06
Correspondence: LI You-ji, PhD Candidate; Tel: +86-13787118865; Fax: +86-731-4573166; E-mail: bcclyj@163.com